Open PDF in Browser: Casey J. Nelson,* A First Amendment Failure: Surrendering to Science Misinformation for Bioengineered Foods
Government-compelled commercial disclosures are not unfamiliar to consumers. Common labels include nutrition facts and ingredient information. The National Bioengineered Food Disclosure Standard, which took full force at the start of 2022, is of a different nature. The new law requires all manufacturers, all importers, and certain retailers of bioengineered foods to disclose on the food’s packaging that it has been produced with bioengineering technology. Even so, a large swath of the public is ignorant of “bioengineering’s” true meaning and bioengineering technology’s true quality. The politically charged and fact-lacking debate on bioengineered foods renders this standard an impermissible coercion of speech in violation of the First Amendment. If stricter regulation of potentially harmful food products is truly desired, a more appropriate target for compelled disclosure is pesticide use. Using science communication principles and factual information on the outcomes of bioengineered foods as a backdrop, this Note argues against the labeling of bioengineered foods and for alternative pesticide disclosures.
Introduction
Humans have cultivated agricultural crops for millennia. Though Charles Darwin coined the term “artificial selection” in 1859,[1] there is evidence of selective crop breeding in domestic wheat varieties as far back as 7800 BCE.[2] Generations of farmers have gained experience in selecting desirable crop traits and have identified techniques and technologies for crop selection. Farmers commonly aim to maximize yield and minimize cost, thus increasing the general growth and productivity of their farms.[3] While farmers have consistently held the goals of yield maximation and cost minimization, they now rely on modern bioengineering technology to achieve these goals more easily.[4]
The rapidly advancing field of genetics has contributed to the agricultural industry since the field’s inception.[5] Early food scientists hoping to tackle food crises or maximize cost-effective production worked diligently to select certain advantageous traits in crops while cutting out other, less desirable traits.[6] This selective genetic breeding process was originally lengthy and inconsistent, yet it was still far more reliable than nonbioengineered gene selection techniques such as crossbreeding flourishing plants.[7] Not long after undergoing its first dramatic change thanks to genetics research, the agricultural industry experienced a second genetics breakthrough.
In 2012, Dr. Emmanuelle Charpentier and Dr. Jennifer A. Doudna made a Nobel Prize-winning discovery that changed the field of genetics forever.[8] Agricultural geneticists were already working on specialized seeds, and researchers in medicine were already studying genetic cancer treatments—but the cutting-edge CRISPR-Cas9 “genetic scissors” technique expedited virtually all genetic research.[9] The new technique allows scientists to cleave genetic material at exact, predetermined sites using efficient guiding markers.[10] As a result of this discovery, scientists can now make precise genetic changes, expediting research on inherited diseases, certain cancers, and crop durability, ultimately “bringing the greatest benefit to humankind.”[11]
However, not everyone was buoyed by the promise of efficient genetic editing. Although public campaigns against bioengineering have been around for as long as scientists have worked in the genetics field,[12] negative media coverage of bioengineered crops ballooned after the discovery of genetic scissors.[13] Freshly fueled opponents of bioengineering cite fears including allergic reactions and illnesses, environmental risks, and “unnaturalness.”[14] For example, the Non-GMO Project lists the following as elements of its mission: protecting “[t]he integrity of our diverse genetic inheritance”; “reducing contamination pressure and protecting the supply of non-GMO seed”; “transitioning toward a non-GMO food supply”; and consumer choice.[15] Antibioengineering interest groups have saturated public dialogue with misinformation through traditional and social media,[16] much like other antiscience groups that target evolution, climate science, and vaccines.
In line with an unfortunate modern trend, science communicators struggle to persuade consumers that the oft-repeated ill effects of bioengineered foods are unfounded.[17] This Note offers a glimpse into the study of science communication, a study that attempts to determine how to best relay scientific information to nonscientists, in an effort to explain consumer hesitance on bioengineered foods. Science communication theories buttress the argument that antibioengineering stances are not based on facts but on mental shortcuts and social phenomena. Government-compelled speech has no place in such a distorted debate.
The Biden Administration has embraced emerging biotechnology in the face of negative media portrayals of bioengineered foods. President Joseph Biden signed an Executive Order that has instead placed an optimistic national spotlight on bioengineering practices.[18] The Order establishes a National Biotechnology and Biomanufacturing Initiative that emphasizes American innovation, sustainability, biosecurity, ethics and responsibility, and economic strength.[19] Food and agriculture are not the only implicated fields—the Order also refers to the “vital role of biotechnology and biomanufacturing in developing and producing life-saving diagnostics, therapeutics, and vaccines” in light of the COVID-19 pandemic.[20] But the Order’s positive stance on and encouragement of biotechnology is not the only recent governmental response to consumer buzz.
The pervasiveness of misinformation about bioengineered foods has led to federal legislation requiring their labeling.[21] In spite of the Executive Order’s optimism, today’s antibioengineering movement has gained enough momentum to instigate the passage of the National Bioengineered Food Disclosure Law (also referred to as the National Bioengineered Food Disclosure Standard or “NBFDS”) in 2016.[22] The law passed despite over one hundred million dollars spent lobbying against it on behalf of powerful food, farming, and biotechnology corporations.[23] The NBFDS mandates the disclosure of “any bioengineered food and any food that may be bioengineered”[24] via “text, symbol, or electronic or digital link” on food packaging.[25] In grocery stores, this appears as text that states “bioengineered foods” or “bioengineered food ingredients,” a government-created “bioengineered” symbol, a qualifying electronic or digital link, or an informational text message disclosure.[26] This Note argues that the lack of nuance and absence of valuable decision-making information in these disclosures do more harm than good for consumers. Practically speaking, a “bioengineered” food label may either confuse consumers or stoke the fire in a heavily misinformed public debate.
Information on food labels easily qualifies as commercial speech,[27] and government compulsion of commercial speech is controlled by the Supreme Court’s First Amendment doctrine under Zauderer v. Office of Disciplinary Counsel of the Supreme Court of Ohio.[28] The deferential Zauderer test requires a compelled commercial disclosure to contain (1) “purely factual and uncontroversial information” that is (2) “reasonably related to the [government]’s interest in preventing deception of consumers” and (3) not “unjustified or unduly burdensome” on further commercial speech.[29] If any prong of this standard is not satisfied, Zauderer deference is not granted to the government and the more stringent intermediate scrutiny test for commercial speech laid out in Central Hudson Gas & Electric Corporation v. Public Service Commission of New York applies.[30] Under Central Hudson, in order for commercial speech to be constitutionally regulated by the government, (1) the speech must be lawful and not misleading, (2) the government must have a substantial interest in regulation of the speech, (3) the regulation must directly advance the government’s substantial interest, and (4) the regulation must not be more extensive than necessary to achieve that interest.[31]
This Note contends that newly implemented, federally mandated bioengineered food labels are unconstitutional under First Amendment doctrine. While deferential to the government, the Supreme Court’s Zauderer test for compelled commercial speech offers little room for deference toward the NBFDS. Additionally, commercial speech regarding bioengineered food labels will not survive a subsequent intermediate scrutiny Central Hudson test.
In short, bioengineered food labeling does not further the purposes of the First Amendment. NBFDS-compelled speech fails to contribute to—and in fact disrupts—a productive marketplace of ideas because it does not provide valuable decision-making information to consumers.[32] Similarly, the NBFDS does not help the public reach the best and most accurate conclusion in furtherance of a democratic self-governance theory of the First Amendment.[33] In reality, bioengineered foods offer safe, sustainable, secure, affordable, and innovative solutions for the global market as the world adapts to population growth, decreasing fertile land, and climate change. Bioengineered food labeling instead quashes such hopes by perpetuating misinformation and disinformation, harming the economy, and failing to offer any real improvement to consumer choice.
Recognizing the merits of consumer choice, this Note proposes more appropriate mandatory labels that reflect true information about food products and do not invoke a debate steeped in misinformation. Specifically, disclosures about agricultural pesticide use would address the legitimate concerns of consumers by attacking the producers of bioengineered crops who use bioengineering technology to harmful ends. For example, powerful players in the agricultural industry have bioengineered crops that can withstand large amounts of pesticide spray, resulting in significant environmental harms and legitimate health concerns.[34] A subset of bad actors can weaponize any powerful technological tool. But a tool that has such significant capability to serve the greater public interest should not itself be demonized simply because of these actors.[35]
Part I begins with a brief description of genetic scissors as a bioengineering method and lists the method’s remarkable applications—as well as its potential for misuse. The Part then discusses the study of science communication and offers insight as to how public perceptions on bioengineered foods are shaped. Part I concludes with an analysis of the legislative branch’s capture in the antibioengineering movement.
Next, Part II lays out the legal framework for commercial speech and explains the justifications for its protection. This Part uses food- and science-based caselaw to interpret the Zauderer test for compelled commercial speech. In addition, it uses relevant caselaw to explore recent movements and tensions in this test.
Using Part I’s science communication principles as a framework, Part III asserts that the NBFDS cannot be granted Zauderer deference according to two of the test’s three prongs and fails Central Hudson intermediate scrutiny. Finally, Part IV suggests a path forward by eliminating bioengineered food labels entirely, instead opting for labels that target harmful pesticide use.
I. Factual, Data-Driven Implications and Perceptions of Bioengineered Foods
Take a moment to think about what you know about bioengineered foods, often referred to using the umbrella term “GMOs.” Take stock of your factual knowledge, gut reactions, grocery store habits, and whether you even know what the acronym stands for.[36] Next, think on where you may have learned that knowledge or gained those perceptions and habits. How would your perception change if you read a “bioengineered” label on a box of cereal or can of soup? Self-reflection on personal attitudes toward bioengineered foods and other specialized scientific concepts is important in advancing conversations in the greater field of science communication. The forthcoming Part summarizes not only quantitative data and scientifically identified effects of bioengineered foods but also the measurable science behind how public perceptions of bioengineered foods have evolved over time.
Section I.A educates the reader on genetic scissors from a layperson’s perspective. The Section then offers examples of exciting avenues of research with respect to bioengineered foods and explains some negative connotations derived from such foods. Section I.B describes well-researched science communication theories and applies them to public perceptions of bioengineered foods. After learning how laypeople typically form science perceptions, Section I.C sets the stage for how the government became entrenched in legislation that took sides against science.
A. Varying Applications of Bioengineering
The discovery of the CRISPR-Cas9 system, or “genetic scissors,” revolutionized genetic research. In contrast to early bioengineering, which was far more complex, expensive, and limited to one genetic modification at a time, genetic scissors can insert, remove, or replace multiple genes at once.[37] Amplifying genetic traits in crops such as “herbicide resistance, drought tolerance, improved nutritional content, salt resistance, and resistance to biotic stress” is more achievable now than it has ever been because of current technology’s precision and predictability.[38] Using genetic scissors is effective, reliable, and results in fewer unexpected outcomes than previous bioengineering methods.[39] The method’s ease of use and potential for broad applications originally led scientists to predict that the public might be more accepting of bioengineered products made using this technology.[40]
In evaluating both this novel bioengineering method and traditional breeding methods, it is important to note that the genetic modifications made in living organisms do not introduce non-organic materials.[41] Put another way, the genetic code of the organism is being shuffled to produce a certain outcome. This shuffling can be compared to a musical artist using different combinations of chords to produce a cohesive song, with each song contributing to a cohesive album.[42] Using this analogy, only musical chords are combined, and not any nonmusical sounds like glass breaking or a dog barking.
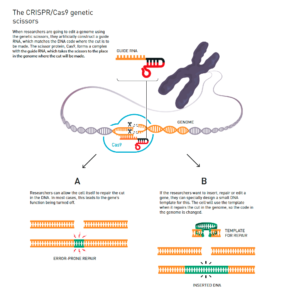
Figure 1: The CRISPR/Cas9 Genetic Scissors[43]
Deoxyribonucleic acid (DNA), the genetic code that exists in all living organisms and dictates what kind of living thing an organism is, is made up of four components: A, C, T, and G building blocks (for short).[44] Bioengineering methods do not introduce any new “letters” or building blocks into an organism—these methods merely add, remove, or switch out different combinations of the same building blocks. Figure 1 above visually depicts how scientists make these changes to DNA. Similarly, four musical chords can be combined in infinite ways to create different songs without introducing any other sound.
In order to make precise changes to the sequence of DNA building blocks, scientists must identify the genes they intend to change and their locations within the organism’s DNA sequence. Accordingly, musicians must identify in which parts of a song they intend to arrange chords, such as in the verse, chorus, or bridge.[45] Beyond these mechanics, scientists must also weigh the implications of their choices to bioengineer certain genes and subjects, just as musicians must weigh their chord choices according to the genre of their composition, such as jazz or rock and roll.
The following two Sections lay out the true value of this technology in the minds of trailblazing scientists—as well as the disappointing route some scientists have chosen while working for companies that prioritize economic outcomes. The Section then concludes by addressing the valid risks of bioengineered crop cultivation if best practices are not followed.
1. Bioengineering as a Means to Benevolent Ends
The medical community immediately recognized the significance of genetic scissors.[46] The Royal Swedish Academy of Sciences, in awarding the Nobel Prize in Chemistry to the scientists behind the discovery of genetic scissors, postulated that the discovery not only opens the door for new cancer therapies but also “may make the dream of curing inherited diseases come true.”[47]
For agricultural scientists, the landscape seems equally boundless. To date, bioengineered crops have primarily focused on traits that would both reduce the need for pesticides and increase crop output, thereby increasing farm productivity and lowering costs for consumers.[48] But using bioengineering methods to produce food, while more affordable than it has ever been, is still not cheap.[49] Thus, producers may face pressure to maximize economic benefits during the research-and-development phase of bioengineering modern crops.
Still, producers of bioengineered foods have achieved a variety of accomplishments in the field. First, bioengineering methods have significantly reduced the need for pesticide spraying during agricultural cultivation. Pesticides are products that kill insects, rodents, plants, and fungi, largely by way of chemicals that are toxic to those organisms.[50] These products can negatively impact soil quality, farmer health, and surrounding ecosystems and organisms, including pollinators.[51] In an effort to avoid some of these negative outcomes, food scientists have accomplished alternative pest control methods by inserting a gene found in common bacteria into crops such as corn.[52] When a preying insect tries to eat a bacterium that possesses this gene, the gene allows the bacterium to mount a defense against the insect: the bacterium will naturally release a protein that is toxic to the insect and physically breaks the insect down.[53] Crops that have been bioengineered to possess this gene can similarly break down their own preying insects.[54]
The insect-harming protein has never been found to be toxic to humans, and crops that have been bioengineered in this way have actually brought about positive health and environmental effects due to the decreased need for pesticide spraying.[55] However, it is important to address the concerns regarding the potential unintended effects of these crops on an ecosystem level. While scientists continue to explore these effects, research has emphasized that critical pollinators have not been impacted and genetic scissors may actually provide strategic avenues to avoid nontarget effects.[56]
A second valuable outcome for bioengineered crops is their potential for health benefits, as exemplified in the bioengineered crop known as Golden Rice. The rice was developed to reduce vitamin A deficiency for millions of people in developing countries who suffer from the effects of this deficiency: blindness and infant mortality.[57] Unfortunately, uphill regulatory battles have restricted and delayed the planting of Golden Rice because of its bioengineered nature.[58] The battles stem from concerns about the unintended effects of the bioengineered crop.[59] The resulting delay prevented saving an estimated 2.6 to 10.2 million people from eyesight loss over thirty years.[60] In 2021, the Philippines became the first, and remains the only, country to approve cultivation of the grain.[61] Even when a bioengineered food product can directly serve a net good, governments and those they serve remain skeptical of bioengineered foods globally.[62]
Third, bioengineered foods have encouraging signs with respect to biodiversity. Because many crops reproduce via airborne pollen, some groups are concerned about the potentially broad reach of bioengineered traits in crops that mate with wild species.[63] While these concerns are logical, there is little evidence to support that bioengineered crops currently threaten or negatively affect overall biodiversity today.[64] Additional fears have arisen about the potential for bioengineered crops to dominate the market, thereby making national or global food supply vulnerable to a single disease outbreak.[65] The infamous Irish potato famine resulted from such genetic monoculture.[66] Notably, fears about decreased genetic diversity were also raised during the “green revolution” in the 1970s with the wide distribution of traditionally bred crop varieties, but those fears have not been substantiated.[67] In fact, bioengineering methods may offer unique opportunities to preserve failing crop varieties that may otherwise be lost[68] or even revive species that have been decimated by the effects of climate change.[69]
Fourth, bioengineered crops are an important tool for climate change mitigation because they reduce greenhouse gas emissions.[70] Climate change poses unique problems for today’s farmers—the amount of fertile, arable land has drastically decreased, water shortages are common, natural disasters are far more frequent, and changes to the ecological landscape threaten all life forms.[71] These novel problems require novel solutions.
On the mitigation side, basic crop yield increases that are accomplished via bioengineering methods “can reduce the need to add new land into production . . . .”[72] Less overall land use translates to lesser need for emissions-costly agricultural land use changes.[73] Certain bioengineered crops also “support carbon sequestration in the soil by facilitating reduced tillage farming.”[74] Carbon sequestration mitigates climate change by reducing the amount of carbon dioxide in the atmosphere via its capture and storage elsewhere.[75] “Globally, the shift to no-till practices that resulted from the adoption of [bioengineered] herbicide[-]tolerant crops is estimated to have led to the sequestration of soil carbon equivalent to 17.6 Mt [metric tons] of carbon dioxide.”[76] Additionally, the National Research Council has found that farmers’ adoption of bioengineered crops is complementary to conservation-based tilling practices.[77]
In addition to mitigation, bioengineering methods can solve adaptation problems connected to climate change. As previously mentioned, crops can be bioengineered to withstand increasingly extreme environments brought on by climate change.[78] Examples of difficult growing environments may include high winds, flooding or droughts, harsh or fluctuating temperatures, and even increased carbon dioxide levels.[79]
Finally, bioengineered crops provide farmers in developing countries with vital tools for agricultural success. These farmers may have fewer options for pest management, making their crops more vulnerable to disease outbreaks.[80] Thus, the reduced need for pesticides greatly affects their output.[81] Additionally, the landscape of U.S. regulation of bioengineered foods may have direct implications beyond U.S. borders. For example, because of existing trade relationships, strict regulation of bioengineered foods in Europe in turn affects regulatory efforts in African nations.[82] In 2016, “[t]he Information Technology and Innovation Foundation (ITIF) estimate[d] that the current restrictive climate for agricultural biotech innovations could cost low- and lower-middle-income nations up to $1.5 trillion in foregone economic benefits through 2050.”[83] The 2020 implementation of the NBFDS surely did not improve the landscape for farmers of a similar economic background who participate in the U.S. economy.
The introduction of genetic scissors has further allowed food scientists to explore valuable public interest-based aspirations, such as reduced pesticide use and consumer costs, increased nutritional value, environmental conservation, and global economic development. Importantly, bioengineered crops also contribute significantly to reductions in greenhouse gas emissions. In addition to the broad scientific consensus on the safety of bioengineered foods,[84] bioengineered foods are “endorsed by the American Medical Association, the National Academy of Sciences, the American Association for the Advancement of Science and the World Health Organization . . . .”[85] Recall that the scientific advancement of genetic scissors is not confined to agricultural purposes, but it also creates boundless opportunities in medicine and pharmaceuticals. Yet genetic scissors as a means still have the potential to be used for less benevolent ends—in some cases, unethical or avaricious ends.
2. Undesirable Applications by Powerful Commercial Actors: Monsanto
As in many industries, the agricultural industry has both good and bad actors. In contrast to inserting pest-fighting genes, as previously mentioned,[86] food scientists may instead opt to insert pesticide- or herbicide-resistant genes into bioengineered crops. Monsanto, a large agrochemical company acquired by Bayer in 2018, did just that.[87] Monsanto’s introduction of “Roundup Ready” bioengineered crops allowed farmers to spray herbicides without destroying the crops themselves.[88] Initially, the aim of this bioengineered resistance was to use far less herbicide than common practice, thereby making these bioengineered crops safer for farm workers and for human consumption.[89] Moreover, positive environmental effects were associated with these bioengineered crops because the decreased need for tilling preserves soil health.[90] Monsanto’s choice to bioengineer their crops for herbicide resistance also specifically encourages farmers to buy not just Monsanto’s seeds but also its herbicide, Roundup.[91]
Notwithstanding the goals of this practice, complications quickly emerged. Certain weeds developed resistance in response to the overuse of Roundup herbicide by farmers.[92] The uptick in “superweeds” then led farmers to increase their use of herbicides further still.[93] In turn, downstream ecosystems were harmed by the toxic amount of herbicide runoff, and there is now heightened concern surrounding the amount of residual herbicide that is safe to consume with respect to these products.[94] Moreover, because of Roundup’s emerging weed resistance, Monsanto has released a less weed-resistant, more environmentally harmful herbicide called dicamba.[95] Perhaps because of the company’s economic success, Monsanto has never displayed concern with or taken ownership of the destructiveness of its products.
As some farmers praise the invention of Roundup Ready crops because of their ease of use, Monsanto’s hold over the industry tightens.[96] Those farmers who resist growing Monsanto seeds due to environmental damage by herbicides have reported “feeling bullied into” adopting Monsanto seeds.[97] Simply put, if all of your neighbors are using harmful herbicides and your farm is suffering from it, the easy solution is to buy Monsanto’s herbicide-resistant seeds.[98] Moreover, Monsanto and its successor, Bayer, have lost several civil suits brought by plaintiffs claiming that Roundup causes cancer[99]—most recently, Bayer was ordered to pay $2.25 billion to a single plaintiff in January 2024.[100] In the face of the discovery of Roundup’s carcinogenic effects, Bayer maintains that Roundup is safe to use and has assured that the product will stay on shelves.[101] The only business change that Bayer has announced relates to the transparency of its safety research, likely as a result of court documents that revealed that Bayer sought to influence powerful government regulators and shape scientific research.[102]
Importantly, the concerns surrounding herbicide-resistant bioengineered crops have little to do with the crops being bioengineered and much to do with how bioengineering methods are applied in practice. Bioengineered herbicide resistance does not have the same purely public goals as crops such as Golden Rice, and adjacent private goals may encourage commercial actors to overlook negative outcomes.
3. The Importance of Employing Best Practices in Bioengineering
Some consumers claim that bioengineered foods cause allergic reactions.[103] This line of thinking originated from an accidental instance of cross-pollination between a bioengineered corn that was never intended for human consumption and a traditionally bred corn that was intended for human consumption.[104] The bioengineered corn had similar insecticidal properties as mentioned previously,[105] though it was not intended for human consumption because of its low digestibility and potential for allergic reaction.[106] After a few dozen people reported potential allergic reactions from the accidentally cross-pollinated corn product, the product was recalled.[107]
Upon studying the twenty-eight people who reported allergic reactions connected to the corn product, the CDC found that “none of the CDC-submitted samples reacted in a manner consistent with an allergic response” to the protein expressed by the bioengineered gene.[108] The CDC suggested that the subjects may have had allergic reactions, but it was ultimately unable to trace the reactions back to the bioengineered gene.[109] However, the CDC noted the difficulty of allergy testing while emphasizing that such tests are important in the discussion of bioengineered foods.[110] No allergic outbreaks have been linked to bioengineered foods since this singular unconfirmed instance of allergic reaction in twenty-eight people nationwide.[111]
It is worthwhile to reiterate that the bioengineered gene was known by scientists for its potential to cause allergic reactions, and the bioengineered crop was not intended for human consumption. Thus, while the cross-pollinated corn that was used for the corn product may have absorbed a potentially harmful gene, the danger of this product did not stem from the use of bioengineering itself but from inattention to known farming practices.[112] Farmers can effectively and safely manage cross-pollination via practices like staggering planting dates, adapting to prevailing wind directions, and, most easily, physical crop separation.[113]
Unintended cross-pollination is a valid concern for farming generally. However, the risks of cross-pollination can easily be managed by maintaining physical distance between crops.[114] Some crops mate far more readily than others; recommended separation distances vary as much as 660 feet for corn to just 10 feet for soybeans.[115] Though cross-pollination is a risk for certain farmers who may want their products to remain organic, there are no inherent risks to human or environmental health associated with cross-pollinated bioengineered crops, just as there are no such inherent risks associated with bioengineered crops by themselves.[116] Much like in the above corn product scenario, any risk of an unsafe final product depends on the likelihood of cross-pollination with other plants that express unsafe traits.
Genetic scissors enable such precise gene management that any kind of property, including allergenic properties, can theoretically be inserted on purpose. Of course, bioengineered food focuses on creating effects that are not detrimental to humans, and the same strict regulations apply for bioengineered food crops as all other foods.[117] In conclusion, no intrinsic health or environmental risks have been proven with respect to bioengineered food consumption or production, and the impacts of bioengineered crops depend largely on the goals of the bioengineered crop. So, how did large swaths of the public come to sincerely believe the purported evils of bioengineered foods? The following Section turns to this question.
B. How Public Perceptions Are Shaped
Science can be communicated in a variety of ways, some more effective than others. The public can learn about scientific topics in formal settings such as classrooms but also in informal settings such as parks, science museums, local events, or through the media. The study of effective science communication emphasizes the importance of educating the public on science topics through informal means.[118] At the same time, it highlights the need for scientists to “understand and address the perspectives of interest groups, policy makers, businesses, and other players in debates over decisions that require scientific expertise.”[119] In short, conversations between scientists and the lay public should ideally be just that—a conversation.
This is not an easily accomplished task. The absence of two-way communication has left gaps in scientific knowledge and understanding for some members of the public.[120] Misunderstanding can lead to widespread misinformation, which has recently been in the news in relation to how pandemics spread, the efficacy and safety of vaccinations, and other public health measures.[121] Misinformation in the science sphere can have dangerous impacts. For example, about one-third of all COVID-19 deaths in the United States have been recorded by people who remained unvaccinated after vaccines were available to all adults.[122] The existence of evolution, climate change, and even the solar system have seen public waves of doubt in modern history. Though this Note focuses on the science and perceptions of bioengineered foods, the glaring issue of effective science communication reaches far beyond this scope.
After discussing demonstrated science communication findings about how public perceptions of science topics are formed, this Section summarizes the current mainstream perceptions of bioengineered foods.
1. The Study of Science Perceptions
Initially, researchers studying public perceptions of science relied on the “knowledge deficit model.”[123] This theory, now widely rejected, maintained that if the public simply had more factual knowledge of scientific concepts, public acceptance would follow.[124] Of course, this reveals a certain arrogance that can pervade among scientists and appears to lay blame on the public for its ignorance. At the same time, the model does not accurately predict positive public opinion once factual knowledge is gained.[125] Instead, researchers have found that a layperson forms an opinion on a scientific topic using “both scientific facts and non-scientific beliefs, specifically values.”[126] Because a layperson may have neither the time nor the will to educate themselves, it actually “makes perfect sense for [them] to rely on shortcuts . . . .”[127]
These shortcuts can take many forms and have varying effects on perceptions. The “spiral of silence” theory proposes that a layperson who is not factually knowledgeable on a subject may feel pressured by the “loud” majority so as to avoid “being on the losing side of a public debate.”[128] In essence, a person will assess the room and take the majority position for fear of appearing uninformed, and they often stubbornly stick to this position for the same reason.
Framing also plays a large role in shaping perceptions in media coverage, advertising campaigns, and political messaging.[129] Factors that play a role in the persuasiveness of framing include consistency with personal values, alignment with the greater audience’s values, connection to personal experiences of the audience, credibility of the framers, and the portrayed narrative.[130] For example, a media article’s successful frame would emphasize a factor that its readers would identify with, such as their consumer identity or level of civic involvement. In reality, this may look like a news article appealing to a young adult by using a social media screengrab to accompany its linked article. Or, an article may emphasize a “local” environmental issue to engage with politically active readers in the community.
2. Current Antibioengineering Perceptions
While medical genetic breakthroughs are often heralded as just that, advances in the food industry are treated quite differently in the public eye.[131] In fact, data shows that the viewpoint that bioengineered foods pose a “serious health hazard” is on the rise in the United States.[132] Another well-known consumer concern is a moral one, having to do with the “unnaturalness” of bioengineering technology in the food industry.[133] This moral viewpoint is certainly represented, but it has recently been proven to be less relevant than other factors, such as the notions that bioengineered foods only benefit manufacturers and that they cause allergy or illness.[134] Interestingly, “potential benefits [of bioengineered foods] appeared to be less salient to [survey] respondents in influencing rejection . . . .”[135] In effect, negative portrayals of bioengineered foods resonate more with a layperson than positive portrayals, potentially due to the greater and enduring impact of risk-based information on consumers.[136] Strong negative perceptions of bioengineered foods may have roots in risk-based information, though certain demographic factors also play into perceptions.
Several factors have been proven to contribute to the formation of individual scientific perceptions. Studies have demonstrated that “age, conservative political ideology, and greater deference to scientific authority predicted less concern with [bioengineered] foods,” while women, people with children, and “the food conscious” correlated with more concern.[137] Additionally, paying attention to the media’s coverage of bioengineered foods is a strong indicator of negative bioengineered food perceptions, where a recent study noted that “[t]hose who did not pay attention to the media . . . were less likely to express concern with [bioengineered] foods, regardless of perceived familiarity.”[138] This finding alone reveals the severely negative role that the media plays in shaping public perceptions against bioengineered foods.
As the spiral of silence theory suggests, perceptions built on shortcuts, such as what others think or what the media overwhelmingly relays, can create a kind of feedback loop. The nature of today’s traditional and social media coverage can exacerbate this phenomenon, where algorithms create echo chambers and exaggerate what a layperson may view as the majority public opinion.[139] In effect, it is difficult to dig out of the hole of misinformation.
C. Resulting Government Regulation: First States, Then the NBFDS
Unfortunately, the government has now fallen into this hole. After over twenty years of genetically modifying crops without major public pushback, the controversy was resurrected in the 2010s.[140] The anti-bioengineered-food movement gained traction, and citizens in the northeastern United States became the first to take successful action.[141] Connecticut and Maine passed bioengineered food labeling measures, though both laws had trigger clauses where the labeling law only applied after nearby populous states passed similar laws.[142] Vermont became the first state to enact a sweeping mandatory labeling law without such a trigger clause.[143] While lawmakers in Connecticut and Maine were concerned about the potential economic disadvantages of mandating bioengineered food disclosures,[144] lawmakers in Vermont were energized at the thought of being the first to do so.[145]
In addition to local pride, as in Vermont’s case, studies have shown that legislative pushes proved far more persuasive and likely to result in success when the issue of bioengineered food labeling was framed in connection with identity.[146] This type of framing appealed to “a ‘consumer’s right to know’ and used key messages like ‘informed decision’ and ‘consumer choice’ that linked to other master frames like individualism.”[147] For citizens in Vermont, identifying with the narrative of their agricultural state also proved important, where labeling proponents pushed their state as one that “supports sustainable, agroecological farming practices.”[148]
Because similar antibioengineering movements were gaining traction in several states across the country, Congress stepped in and passed legislation to implement the National Bioengineered Food Disclosure Standard (“NBFDS”) in 2018.[149] Through the NBFDS, Congress aimed to implement a “uniform national standard . . . to prevent a patchwork of state, tribal, and local requirements.”[150] In line with this motivation, the legislation expressly preempts any state labeling standard.[151] The NBFDS, implemented by the Secretary of Agriculture, requires food products “contain[ing] genetic material that has been modified through in vitro recombinant deoxyribonucleic acid (rDNA) techniques and for which the modification could not otherwise be obtained through conventional breeding or found in nature” to either bear the label “bioengineered foods” or “bioengineered food ingredients”; an approved “bioengineered” symbol; a qualifying electronic or digital link; or a number to text message to receive disclosure information.[152] The legislative history suggests that the logic behind the labeling mandate is allowable and evenhanded because it “treat[s] a bioengineered food the same as its non-bioengineered counterpart.”[153]
However, the existence of such a requirement for bioengineered foods stands counter to the idea of equal treatment. If Congress truly treated the safety of bioengineered foods no different from nonbioengineered foods, there would be no need for such a disclosure. The mere presence of a disclosure itself suggests that there is some danger associated with the bioengineered food, similar to the Sixth Circuit’s reasoning in striking down a dairy hormone disclosure in International Dairy Foods Association v. Boggs discussed infra.[154] Thus, the NBFDS itself props up a false narrative.
Moreover, antibioengineering and pro-labeling actors have pushed for the labeling of bioengineered foods for years, and they have employed fearmongering tactics every step of the way.[155] Though they purport to advocate for the public’s interest and its “right to know,”[156] these groups have agendas steeped in misinformation that run counter to the true value of bioengineered foods.[157] Antibioengineering groups do not share the same reasoning as Congress that the labels are “designed solely to address marketing matters” or that bioengineered foods and nonbioengineered foods are treated equally under this law.[158] In fact, the founder of the Institute for Responsible Technology, an explicitly antibioengineering organization,[159] stated after the NBFDS’s passage: “Labeling GMOs was never the end goal for us. It was a tactic” in the “more important effort to eliminate GMOs from the market altogether.”[160]
Perhaps unsurprisingly, the motivations behind some groups’ pushes for labeling have been exposed as economic. The Organic Consumers Association, on a self-reported mission to “protect and advocate for consumers’ right to safe, healthful food,”[161] campaigned for a California ballot initiative that would label bioengineered foods.[162] In doing so, the organization pointed to labeling as the quickest way to move organic products “from a 4.2% market niche, to the dominant force in American food and farming[.]”[163] Another pro-organic organization that advocates for the consumer’s “right to know” is the Center for Food Safety.[164] The Center’s executive director has stated, “We are going to force them to label this food. If we have it labeled, then we can organize people not to buy it.”[165]
It is unfortunate that the federal government, let alone the public, has fallen prey to this movement that employs deceptive tactics to meet ulterior economic ends. While Congress cites consumer choice and uniformity as its reasons behind the NBFDS, this Note argues that the NBFDS is a baseless law that achieves only a fraction of these objectives.
II. Historically Commercial Speech and the Application of the Zauderer Test
Shifting to First Amendment principles, this Part parses out the rather muddled doctrine of compelled commercial speech. Section II.A first provides an overview of the parameters of commercial speech, then Section II.B follows by exploring the three-pronged Zauderer test for government-compelled commercial speech. Finally, this Part closes with a discussion of the resolved and current tensions in applying the Zauderer test and, thus, granting Zauderer deference.
A. Defining Commercial Speech
The Supreme Court has spent the past 106 years attempting to flesh out the breadth and boundaries of the First Amendment’s freedom of speech.[166] The Court’s wrestling with commercial speech has followed similar deliberation. Initially, in the 1942 decision of Valentine v. Chrestensen, the Court considered for the first time whether the Constitution protected commercial speech in the form of an advertising leaflet.[167] Here, a local ordinance prohibited the distribution of commercial information in the streets,[168] and the plaintiff sued the city to enjoin it from enforcing the ordinance.[169] The Court held for the city and found that the Constitution does not protect commercial speech, instead ruling that the regulation of such speech was for legislatures to decide.[170]
However, the Court’s stance on commercial speech protection has shifted over the years. By 1976, at least some aspects of commercial speech were recognized as protected by the Court in Virginia State Board of Pharmacy v. Virginia Citizens Consumer Council, Inc.[171] In that case, a state law prohibited pharmacists from publishing, advertising, or promoting prescription drugs.[172] In its decision, the Court examined the citizens who would be harmed most by this law—those who could not afford to easily change pharmacies if price-gouging tactics occurred.[173] Because of these pragmatic effects, the Court struck down the state law and “conclud[ed] that commercial speech, like other varieties, is protected . . . .”[174]
Commercial speech regulation found its outer boundaries in Central Hudson Gas & Electric Corp. v. Public Service Commission of New York.[175] In Central Hudson, the Court first summarized how to identify commercial speech from a variety of cases.[176] The Court stated that commercial expression is “related solely to the economic interests of the speaker and its audience.”[177] Thus, protecting commercial speech is as much about protecting listeners as it is about protecting speakers. Consumers—listeners—have a social interest in commercial information in the sense that they should be able to make their own informed decisions without paternalistic interference from the government.[178]
The theory that more commercial speech is always valuable aligns with the “marketplace of ideas” principle, which is widely recognized as a preeminent purpose of the Free Speech Clause.[179] Additionally, commercial speech will receive more protection if the speech is valuable to consumers.[180] Government regulation is justified where the commercial speech is “more likely to deceive the public than to inform it . . . .”[181] Identifying commercial speech is straightforward in most scenarios; food and beverage labels are one clear-cut example of commercial speech.[182] However, it is important to recognize that the line between commercial and noncommercial speech can also be blurred.[183]
Returning to Central Hudson, the Court has settled on a controlling doctrine that allows the government to regulate commercial speech using an intermediate scrutiny test.[184] The test is set forth as follows:
For commercial speech to come within [the First Amendment’s Free Speech Clause], it at least must concern lawful activity and not be misleading. Next, we ask whether the asserted governmental interest is substantial. If both inquiries yield positive answers, we must determine whether the regulation directly advances the governmental interest asserted, and whether it is not more extensive than is necessary to serve that interest.[185]
This four-part test is now consistently applied in the judiciary when the regulation of commercial speech is challenged, such as in trademark regulation,[186] roadside signs for employment,[187] or lawyers’ advertisements for legal services.[188]
B. Compelling Commercial Speech
When the nature of the commercial product demands it, the government has required that important disclosures be relayed to consumers. Governments implement these disclosure requirements to protect consumers from deception and minimize confusion[189] as well as to protect human health and the environment.[190] Perhaps the most common instances of compelled disclosure are for tobacco products, basic nutritional labeling, and prescription-drug advertising warnings.[191] However, not all mandates are so straightforward.
In Zauderer, the Supreme Court set forth a three-part test to define instances where the government may compel commercial speech. First, the compelled disclosure must contain “purely factual and uncontroversial information.”[192] This element hinges on the information’s value to consumers and not the speaker’s interest in not speaking.[193] Second, the disclosure requirement must be “reasonably related to the [government]’s interest in preventing deception of consumers.”[194] Finally, the disclosure requirement may not be “unjustified or unduly burdensome” so as to chill commercial speech.[195] If a disclosure passes all three prongs of the test, Zauderer deference is granted and the disclosure is deemed constitutional.[196]
The Court outlined the Zauderer test as it considered an Ohio disciplinary rule for attorneys that required the disclosure of the contingent-fee calculation if a contingent-fee rate was mentioned in the advertisement.[197] Because a layperson is unable to decipher the weight and meaning of these calculations and because the likelihood that consumers would be misled by deceptive commercial speech in this realm goes well beyond speculation, the Court upheld the disclosure requirements.[198] Since this decision, the Court has reiterated that the Zauderer test should be applied where the government “impose[s] a disclosure requirement” that is “directed at misleading commercial speech.”[199]
Over the following two Sections, this Note discusses the learning curve that courts have experienced with applying Zauderer, and then it examines the test’s remaining tensions.
1. Evolution of the Zauderer Test via Food and Science Examples
Though inconsistent in how it is applied, courts have consistently relied on Zauderer to determine whether government-compelled commercial speech passes constitutional muster. Compelled speech was permissible in National Electrical Manufacturers Association v. Sorrell, where the Second Circuit considered the constitutionality of a Vermont labeling law that required certain manufacturers to inform consumers that their products contained mercury and should be disposed of as hazardous waste.[200] Instead of applying a strict three-part test, the court used a lenient means-ends analysis pulled from Zauderer to find the required disclosure to be factual and uncontroversial.[201] The court also did not identify any burdens on speech.
Regarding the second step in a three-part analysis—whether the disclosure is reasonably related to the government’s interest—the court relied on Zauderer and other cases.[202] Instead of strictly following the prevention-of-consumer-deception language in Zauderer,[203] the court identified a new valid interest: “protecting human health and the environment from mercury poisoning . . . .”[204] Here, the State’s interest did “not offend the core First Amendment values of promoting efficient exchange of information or protecting individual liberty interests.”[205] The court reasoned that the risks of “forcing speakers to adopt disagreeable state-sanctioned positions, suppressing dissent, confounding the speaker’s attempts to participate in self-governance, or interfering with an individual’s right to define and express his or her own personality” are not great when the government is compelling factual and nonmisleading commercial speech.[206]
While the Sorrell analysis is valuable for understanding the overarching principles of the standard, the Second Circuit has since walked back this rather unstructured approach and returned to the three-pronged Zauderer test in International Dairy Foods Association v. Amestoy.[207] In Amestoy, the court ruled on another Vermont labeling law that required the disclosure of a dairy product’s use of bioengineered hormones in cow milk production.[208] Reasoning that (1) the hormone appears naturally in cows, and (2) there is no scientific evidence that this hormone has any effect on products, the court held that compelled disclosure was unjustified.[209] While the court was “sympathetic to the Vermont consumers who wish to know which products may derive from [hormone]-treated herds, their desire is insufficient” to compel the commercial speech of dairy manufacturers.[210] The court emphasized that “consumer curiosity alone is not a strong enough state interest to sustain the compulsion of even an accurate, factual statement.”[211] Otherwise, there would be no limit to the government’s power to compel production-related disclosures, and any food trend could invade a commercial speaker’s First Amendment rights if it garnered enough support in the legislature.[212] Thus, the required bioengineered hormone labels were not reasonably related to a valid government interest, rendering deference inapplicable and the disclosure unconstitutional under Zauderer’s second step.
In a similar case fourteen years later, the Sixth Circuit ruled on an Ohio compelled-disclosure law involving the same dairy hormone.[213] The law required dairy processors who voluntarily labeled their products “from cows not treated with [hormones]”[214] to also include a statement that there is “no significant difference” between consuming milk from cows treated with the above hormones compared to cows not treated with hormones.[215] The court held that compelled speech in this instance was reasonably related to thwarting the risk of deception.[216] The court classified the voluntary disclosures as “potentially misleading because they imply that conventional milk is inferior or unsafe in some way,” and it concluded that the Zauderer test controlled the court’s analysis for the accompanying disclosure.[217] The court acknowledged that, even though the FDA found “no measurable compositional difference between the two [milks],” there are actually increased levels of a cancer-causing growth-factor hormone in milk derived from hormone-treated cows.[218] Regardless, because the FDA had not found conclusive evidence of health risks related to such increased levels, the Sixth Circuit ruled that the “no significant difference” compelled disclosure was appropriate to avoid misleading consumers into thinking that milk from hormone-treated cows was unsafe.[219] Thus, the compelled disclosure passed the Zauderer test and was granted deference. The court placed an emphasis on minimizing the possible deception of consumers from potentially misleading labels even though there are valid, empirical negative effects associated with bioengineered hormone intake.
2. Identifying Remaining Tensions in the Zauderer Test
Courts and scholars are now comfortable with applying Zauderer as a three-part test, though disagreements remain on how to interpret these steps. The foremost disagreement lies in the first requirement of the disclosure to be “factual and uncontroversial,”[220] where courts, including the Sixth Circuit, have dismissed the “uncontroversial” requirement as mere surplusage.[221] Under this view, the first prong simply ensures that the information in the compelled disclosure is truthful, thus equating “uncontroversial” to “undisputed” as to the facts.[222] However, the Supreme Court recently rejected this approach in National Institute of Family & Life Advocates [“NIFLA”] v. Becerra, where “clinics that primarily serve pregnant women” were required by California law to “notify women that California provides free or low-cost services, including abortions, and give them a phone number to call.”[223] The Court specifically stated that Zauderer deference was disrupted on the basis of the disclosure’s controversial subject matter.[224]
Since this clarification, lower courts have followed in separating “factual” information from “uncontroversial” information in disclosures. The Ninth Circuit, for example, regarded a disclosure that comports with the first Zauderer prong as one that “does not force [commercial actors] to take sides in a heated political controversy,”[225] switching from a prior ruling that “‘uncontroversial’ in this context refer[red] to the factual accuracy of the compelled disclosure, not to its subjective impact on the audience.”[226] Thus, NIFLA gives weight to the “uncontroversial” subpart of the first Zauderer prong.[227]
Another point of contention in Zauderer discourse is the meaning of the second prong, which “hold[s] that an advertiser’s rights are adequately protected as long as disclosure requirements are reasonably related to the State’s interest in preventing deception of consumers.”[228] Courts have split on whether the Court in Zauderer recited the case’s own facts, referring to that specific government interest being substantial, or whether the Court meant to narrowly limit Zauderer’s second prong so as to only apply to cases where the government interest is specifically in preventing consumer deception.[229] However, most lower courts have allowed Zauderer deference to apply to government interests outside of the context of consumer deception.[230] This Note examines the government’s interest in preventing consumer deception and a potential government interest in promoting health and safety for the NBFDS in Part III infra.
Finally, lower courts have run into confusion with respect to the correct standard to apply if Zauderer deference is not granted to a government-compelled disclosure. Some jurisdictions have been dismissive of applying another standard at all.[231] However, the most broadly adopted method is to apply the four-factor Central Hudson intermediate scrutiny test for commercial speech after a compelled commercial speech disclosure is not granted Zauderer deference.[232] This Note employs this established scheme. It is worth noting that if a disclosure is not granted Zauderer deference under its low, deferential standard, it surely cannot succeed under a higher level of scrutiny.
III. The NBFDS Cannot Be Granted Zauderer Deference
The NBFDS’s requirement of a “bioengineered food” disclosure to appear on each bioengineered food product label violates manufacturers’ First Amendment rights. The disclosure is wholly controversial and does not have a reasonable relation to the government’s interest in preventing consumer deception—in fact, it contributes to such deception. To be granted Zauderer deference for compelled commercial speech, the government’s required disclosure (1) must be factual and uncontroversial, (2) must be “reasonably related to the [government]’s interest,” and finally, (3) must not place an undue burden on further speech.[233] If any element of this rational basis test is not satisfied, the government’s compelled disclosure is not granted Zauderer deference and must then withstand Central Hudson intermediate scrutiny.[234]
This Part begins by combining science communication research with relevant caselaw to show that bioengineered food labels are far from uncontroversial, thus failing at Zauderer’s first step. Next, this Part posits that instead of furthering the government’s interest in preventing consumer deception, the NBFDS, in fact, frustrates this interest in violation of Zauderer’s second step. This Part briefly addresses whether bioengineered food labels unduly burden further commercial speech. A government-compelled disclosure that is not granted Zauderer deference under the test’s low threshold will likely also fail a heightened-scrutiny standard. Thus, the compelled disclosure is unconstitutional upon the failure of a single Zauderer prong. This Part closes by demonstrating that the NBFDS, in fact, fails Central Hudson intermediate scrutiny.
A. Compelled Disclosure of Bioengineered Foods Is Not Uncontroversial
Bioengineered food labeling under the NBFDS should not be granted deference based on the first prong of Zauderer, where the government-compelled disclosure must be “purely factual and uncontroversial . . . .”[235] In a relevant 2021 case involving the food industry, the U.S. District Court for the Eastern District of California struck down a compelled disclosure with respect to foods containing acrylamide in California Chamber of Commerce v. Becerra.[236] The court held that requiring products that contain acrylamide[237] to bear the warning “known to the State of California to cause cancer” failed at Zauderer’s first step.[238] Because “the warning implies incorrectly that acrylamide is an additive or ingredient,” consumers would not be able to discern the underlying logic of the disclosure and would probably come to incorrect conclusions as a result of the disclosure.[239] Moreover, dozens of studies have failed to link a diet containing acrylamide to cancer in humans, so disclosure in this instance “elevates one side of a legitimately unresolved scientific debate . . . .”[240] The court even emphasized that “[s]tatements are not necessarily factual and uncontroversial just because they are technically true.”[241] This further rebukes the interpretation that the “factual and uncontroversial” prong of Zauderer refers merely to the truthfulness of a statement and mirrors the Supreme Court’s finding in NIFLA discussed supra.[242] Notably, the California Chamber of Commerce v. Becerra decision came in the face of several public health authorities voicing concern about the likelihood of acrylamide to be carcinogenic.[243] Even still, the Ninth Circuit echoed the district court’s reasoning in a similar case, holding the disclosure was misleading and improper.[244]
Requiring the disclosure of a controversial topic directly conflicts with the Zauderer standard.[245] Even though it would be factually true for a bioengineered food product to carry a NBFDS label, this is not enough to fulfill Zauderer’s first prong—the “uncontroversial” language is not mere surplusage.[246] Though the Court ultimately decided the case on other grounds, the Court in NIFLA declared that a California law that required pro-life clinics to share state-sponsored abortion-related information was “anything but” uncontroversial, thus explicitly instructing lower courts against reading the term “controversial” out of the test.[247] The Court ruled that Zauderer deference could not be granted in NIFLA “[m]ost obviously” because the disclosure was of a controversial nature.[248]
A compelled disclosure may also be controversial when it forces speakers to publicly take a stance when they would rather not. Analogizing to NIFLA once more, the government was forcing unwilling clinics to disclose controversial abortion-related information,[249] similar to the NBFDS forcing unwilling manufacturers to disclose controversial bioengineering information. Ensuring that the government-compelled disclosure is both factual and uncontroversial protects against the government “forcing speakers to adopt disagreeable state-sanctioned positions, suppressing dissent, confounding the speaker’s attempts to participate in self-governance, or interfering with an individual’s right to define and express [their] own personality.”[250] A “compelled statement [that takes] sides in a heated political controversy” and forces speech to the detriment of the speaker is controversial.[251]
The topic of bioengineered foods alone is controversial, which precludes a compelled disclosure at Zauderer’s first step. Even before the current polarized state of bioengineered foods, a dissenting judge on the Second Circuit argued against bioengineered food labels, stating that “[g]enetic and biotechnological manipulation of basic food products is new and controversial.”[252] As discussed previously, the topic of bioengineered foods elicits a variety of opinions that often are not based in fact but on media use, personal identity, and moral questions.[253] A person who sees “bioengineered foods” or “bioengineered food ingredients” on a label in the grocery store is automatically engaged in this debate, and their decision on whether to buy the bioengineered product is often steeped in dialogue lacking factual roots. Congress has forced commercial manufacturers to engage in this debate with consumers by highlighting a safe production method that is publicly saturated with inaccuracies.[254] Given the known disagreements surrounding bioengineered foods, the topic of the NBFDS compelled disclosure can appropriately be described as a controversy and thus violates Zauderer’s first step.
Regardless of how one feels toward bioengineered foods, the labeling of these foods can hardly be described as uncontroversial. This is evidenced by the high-priced lobbying strategies for the NBFDS and other labeling movements across the country,[255] which emphasizes the powerful attitudes on each side of the debate. Disclosure is also controversial because it may lead consumers to unintended conclusions, as the court observed in Becerra.[256] Decisions made on nonfactual bases inherently lend themselves to controversial opinions. Additionally, there was far more scientific support for the contention that acrylamide caused cancer than there is for the claim that bioengineered foods cause negative health or environmental effects.[257] Meanwhile, the labeling of the former was invalidated.[258]
However, it is important to acknowledge that the NBFDS disclosure is admittedly of a different nature than one relating to health and safety. Government-compelled warnings, including California’s Proposition 65 and the Surgeon General’s tobacco warning, hold much different weight than the seemingly informational “bioengineered” label. Though the NBFDS disclosure is indisputably factual, the mere disclosure itself cannot be separated from its controversy.
Therefore, the NBFDS cannot be granted Zauderer deference and in turn violates the First Amendment, both because it compels speech on a controversial subject and because the disclosure itself is controversial. As explored in Section I.B.2, public perceptions of bioengineered foods largely stem from preconceived views that are unrelated to scientific factual knowledge yet align heavily with hot-button-issue media attention.[259] Thus, the NBFDS compels commercial speech that is steeped in controversy and debate.
B. The NBFDS Offers No Substantial Government Interest
Zauderer’s second prong offers two points of analysis. First, what relevant government interest is justifiable enough to satisfy the test? As discussed supra, a valid government interest is likely not limited to one of consumer deception.[260] Second, are the goals of the NBFDS reasonably related to the named government interest? This Section first lays out the relevant rules pulled from compelled commercial disclosure cases. Then, it deconstructs any reasonable relationship to a government interest in preventing consumer deception via the NBFDS before finally refuting a reasonable relationship to a government interest in promoting health and safety.
The government’s interest in aiding consumer choice and preventing consumer deception via compelled bioengineered food disclosures is misguided, as the disclosures only serve to further confuse consumers. In order to be reasonably related to preventing deception, “compelled disclosure [must] further some substantial—that is, more than trivial—governmental interest.”[261] When determining whether the evidentiary record supports a finding of consumer deception, it is “adequate to establish that the likelihood of deception . . . ‘is hardly a speculative one.’”[262]
In terms of a reasonable relationship to the prevention of consumer deception, the government’s compelled “[d]isclosures must remedy a harm that is ‘potentially real not purely hypothetical . . . .’”[263] Zauderer’s objective in preventing deception is disturbed when the government cannot “clearly and legally characterize” the product as one that requires a disclosure.[264] Mandating manufacturer disclosure “about a production method that has no discernable impact on a final product,” which has the function of a warning, is impermissible.[265] Disclosures regarding the production process are impliedly misleading because they suggest that the product “is inferior or unsafe in some way.”[266] As stated by First Amendment scholar Professor Robert Post, “compelled commercial speech may affect how persons experience the salience and framing of information.”[267] He elaborates that food disclosures “implicitly signal that members of the public ought to pay attention to their health when purchasing food.”[268] This reasoning begins to sound familiar when applied to a science communication framing theory.[269]
Though the government’s interest in preventing consumer deception is explicit in Zauderer, other interests may also be adequate as long as they are substantial.[270] As the Ninth Circuit put it, “the [government] interest at stake must be more than the satisfaction of mere ‘consumer curiosity.’”[271] In Sorrell, the Second Circuit upheld the government’s compelled disclosure with respect to mercury-containing products as the disclosure aimed to increase consumer awareness and reduce mercury pollution.[272] Even though the court did not identify the prevention of deception as a true goal of the statute, it justified this secondary interest as one that still furthered the purposes of the First Amendment.[273] These core values include contributing to “the discovery of truth and . . . the efficiency of the ‘marketplace of ideas’” as well as the advancement of individual liberty.[274]
In most cases of nonmisleading commercial speech, compelled disclosures pose little risk of encroaching on the speaker’s rights.[275] However, when Vermont compelled the disclosure of a bioengineered growth hormone, the Second Circuit in Amestoy characterized it as a “constitutional intrusion . . . .”[276] Though the three-part Zauderer test was, perhaps incorrectly, not applied, the court relied on the absence of scientific evidence to distinguish bioengineered products from their nonbioengineered counterparts.[277] Consumers’ “right to know” was insufficient to “compel the dairy manufacturers to speak against their will,” and mandating speech in every instance of consumer interest would allow such disclosures to have an unlimited reach.[278]
Congress’s consumer-choice interest in implementing the NBFDS is insufficient and contrary to the government’s interest in the prevention of consumer deception. Compelled disclosure of bioengineered foods is wholly inefficient in informing consumers because consumers do not generally have the knowledge or tools to discern the true meaning behind a label such as “bioengineered.”[279] Simply stating that a product is bioengineered gives no actual insight into the product’s hypothetical health or environmental results, and the compulsion of such a disclosure in fact misleads consumers into concern. Thus, the NBFDS is at odds with a democratic self-governance theory of free speech, which encourages “a better informed citizenry that can make wise voting decisions, thus ensuring the success of democratic self-government.”[280] The government’s interest here is premised on consumer misconceptions with no scientific foundation. Accordingly, the NBFDS does not comport with constitutional values.
Setting aside the government’s interest in the prevention of consumer deception, the government’s interest in the promotion of health and safety likewise has no reasonable relationship to the NBFDS. In contrast to the sweeping substantive evidence to support that mercury pollution is quite harmful to human health and the environment,[281] scientific studies on bioengineered foods have consistently concluded that no discernable harms exist.[282] Much like in Amestoy, bioengineered food manufacturers’ First Amendment rights are being impeded due to the disclosure’s misleading effect and lack of contribution to democratic outcomes. It cannot be overlooked that “right to know” and “consumer choice” campaigns for bioengineered food labeling have come from proponents whose arguments center around the general danger of bioengineered foods with no basis in fact and some basis in economic interests.[283] The NBFDS plays into the perceived harm that has been perpetuated by interest groups and the media and has consequently affected consumers and politicians to the point of impermissible compelled disclosure.
C. No Undue Burden, But Central Hudson Failure
The Zauderer Court, in describing the third prong of the standard for compelled commercial speech, “recognize[d] that unjustified or unduly burdensome disclosure requirements might offend the First Amendment by chilling protected commercial speech.”[284] In a case concerning compelled health warnings on sugar-sweetened beverages, the Ninth Circuit held that a 20 percent size requirement for the warning was unduly burdensome.[285] The court held that the warning could have been half the size and just as effective, and the warning drowned out protected speech.[286] In contrast, a 25 percent size requirement on cigar packaging and 20 percent size requirement on advertising was not unduly burdensome by the First Circuit’s standards.[287]
While the NBFDS cannot be justified by either of Zauderer’s first two prongs, the compelled disclosure likely passes the test’s third prong. The NBFDS must simply be “of sufficient size and clarity to appear prominently and conspicuously on the label . . . .”[288] This inexplicit size requirement does not come close to those implicated in the cases mentioned above. Additionally, the implementation of the NBFDS does not restrict manufacturers from making additional legal claims regarding the bioengineered nature of their foods.[289]
However, an argument can be made that commercial speakers for bioengineered foods must now defend themselves in the impassioned debate on bioengineered foods. In turn, the NBFDS burdens commercial speakers not by chilling their speech but by placing a burden on them to educate the public about a pilloried method of production. As referenced above, this is a hard enough task for scientists;[290] commercial speakers are ill-equipped to engage in such an education campaign.[291] Thus, the NBFDS forces commercial speakers to speak when they would rather not, particularly on a subject where their products have been alienated for skewed reasons. This tension between speakers’ and listeners’ rights represents a common yet important balancing act in First Amendment free speech analyses. Regardless, the argument that the NBFDS burdens additional commercial speech likely does not have a strong enough basis in caselaw to fail a deferential standard.
Though the NBFDS likely passes muster at this Zauderer step, because a failure at any one Zauderer step corresponds to the inapplicability of Zauderer deference overall, the NBFDS should nonetheless be subjected to heightened scrutiny under Central Hudson.
* * *
In sum, NBFDS bioengineered food labels do not pass the rational basis standard of Zauderer and, in turn, fail under Central Hudson’s four-step intermediate scrutiny test.
As previously laid out, constitutional regulation of commercial speech must first concern speech that is lawful and not misleading.[292] The prior Section has adequately demonstrated that a “bioengineered food” label is directly misleading to consumers, as the media and interest groups muddy the dialogue surrounding these foods.[293] The NBFDS fails Central Hudson here, which is sufficient to fail the entire test and render the government action unconstitutional. However, this Note continues with the analysis for illustrative purposes.
Second, the government must have a substantial interest for regulation.[294] The NBFDS’s legislative history has cited the law’s interest as one of consumer choice.[295] It is debatable whether this would qualify as a substantial interest;[296] reasonable minds may disagree.
Third, the regulation must directly advance the government’s substantial interest,[297] in this case, its interest in consumer choice. Here, the NBDFS may directly advance an interest in consumer choice.
However, the NBFDS also fails at the fourth and final step, which maintains that the regulation must not be more extensive than necessary to achieve the government interest.[298] Valuable contributions to consumer choice can be realized more narrowly than the NBFDS by a different disclosure, namely one that states that the harms from a bioengineered product are no different than the harms from a nonbioengineered product. Alternatively, a prudent disclosure for consumer choice related to bioengineering would concern only pesticide use. This approach would survive First Amendment doctrines as detailed in the following Part.
IV. How Alternate Food Labels Can Pass the Test
If Congress wishes to regulate the true apprehensions surrounding the bioengineered food industry, it has the tools to do so. While the NBFDS does not pass constitutional muster, this Part explains how Congress can address the task at hand more narrowly by targeting pesticide use alone. In doing so, the government has an opportunity to flip the script on public misperceptions of bioengineered foods and shed light on their benefits and necessity. An open rejection of the NBFDS and proper labeling, if truly desired by consumers and legislators, would work in the interests of the nation, the world, and the future of bioengineering technology.
Rather than mandating any sort of disclosure about bioengineering, if the government is truly concerned about consumer safety, it should require specific warnings about products that are empirically known to be dangerous. This Part proceeds by asserting that food labeling should focus on pesticide use. It then discusses the effects that pesticide disclosures—not bioengineering disclosures—would have on farmers, consumers, and the field of science.
A. Addressing Real Concerns Proximate to Bioengineered Foods: Pesticide Use
Instead of broad, unexplained labels that do little-to-nothing to inform consumers, compelled food disclosures should focus on the actual concerns of consumer choice: real-world health and environmental concerns. To be granted Zauderer deference, a compelled commercial disclosure must be rooted in a government interest with the right goals and factual support. The primary way to achieve this is to center compelled food disclosures on pesticide use. This would tackle a much larger and more poignant problem that faces producers of bioengineered foods and nonbioengineered foods alike.
Section I.A.2 discussed the hazards of bioengineering pesticide-resistant crops and cited evidence that cultivation of these crops enables weeds to become resistant to pesticides, which in turn increases agricultural pesticide use. This subsequent increased use causes environmentally damaging runoff and, though quantitatively unproven, may increase the level of harmful pesticide residues lingering on bioengineered foods. The effects of pesticide use on farm workers are more clear: the evidence shows that large swaths of farmers and undocumented workers alike experience symptoms ranging from dizziness to gastrointestinal problems to paralysis.[299] While no measurable harms to health or environment have presently been reported with respect to bioengineered food production, pesticide use has been linked to cancer for those who administer its use.[300] Today’s era has also seen the merging of several prominent agribusinesses with significant power and money, and consumers and the government alike are increasingly interested in oversight.[301]
Several options exist for federal government-compelled pesticide disclosures. First, Congress can apply a disclosure requirement to foods produced with a certain high level of pesticide use per acre of farmland. This usage-based regulation would adequately impact large agricultural companies that have high annual revenues—groups that are often the worst offenders when it comes to potentially harmful pesticide use on bioengineered crops.[302] However, these massive, deep-pocketed companies are also the most likely to initiate sustained lobbying or litigation to push back on such a compelled disclosure.
Second, Congress can require a disclosure for all crops that are cultivated in conjunction with pesticides. Such a disclosure would undoubtedly face similar pushback with powerful companies in the industry. Notably, applying this policy to all agricultural pesticide users removes bioengineering from the conversation entirely. This Note has demonstrated that the only true concern of bioengineered foods does not lie with its production method but with associated farming practices. Pesticide use is widespread, and this policy avenue would encourage even further innovation in bioengineering research to limit pesticide use.
Finally, mandating pesticide disclosures can be accomplished administratively. The U.S. Department of Agriculture and the U.S. Food and Drug Administration can promulgate regulations related to the health and safety of pesticide use on crops for human consumption, both at the farm and in the grocery store.[303] This proposal may parallel the U.S. Securities and Exchange Commission’s proposed rule to require public companies to disclose climate-related risks, “including how they are accounting for, and preparing for, those risks, what they are doing to meet any publicly proclaimed climate goals, and what their greenhouse gas emissions are.”[304] In using this scheme as a model, the compelled disclosures relating to pesticide use and practices would not need to appear on food packaging.
There are no prevalent movements for pesticide disclosures on either the federal or state level. Disclosures in the form of warning labels are required on the toxic chemical products themselves,[305] though none currently appear on food packaging.[306] Critics of a labeling approach maintain that mere disclosure essentially “allows legislators to skirt hard regulatory choices . . . .”[307] This critical stance supports stronger overall regulation of pesticide use on the cultivation side—another viable option beyond the scope of compelled commercial speech.
Altogether, pesticide disclosures are factual and uncontroversial, reasonably relate to a government interest in promoting health and safety, and do not unduly burden further speech. Thus, these disclosures pass constitutional muster and may be granted Zauderer deference.
B. Real-World Effects of Replacing the NBFDS
Small-scale commercial food actors may be concerned about potential economic impacts from the aforementioned pesticide disclosures, though they have already been put on notice by the NBFDS. Additionally, if the actors affected most by pesticide disclosures are harming consumers so much that they lose substantial revenue, that may not be a bad result. In truth, the intended outcome of pesticide disclosures would be to mitigate the harm done to the environment, reduce the potential risk to human health, and ensure that actors who engage in reckless pesticide overuse are held accountable.
From a broader perspective, a government repudiation of antiscience legislation could have significant impacts. Not only would the bioengineered food industry thrive, but it may do so in ways that more readily reflect public-interest goals rather than economic ones. Positive bioengineering effects could be in the form of increased yield and lower prices, enhanced nutritional content for underserved communities, improved economic security and mental health for farmers, reductions in greenhouse gases, and more widespread sustainable farming practices, but also simple consumer interests such as enhanced flavor, extended shelf life, and diversified products.
A rejection of the NBFDS by the government would also spark much-needed conversations about science misinformation.[308] Science misinformation can be especially harmful because of its involvement in life-or-death situations as exemplified by the widespread misinformation surrounding COVID-19 vaccination and related public health measures. Other current battlegrounds include those relating to evolution and climate change, each with their own significant impacts on greater society. As science discussions become more difficult, science communication research and its dissemination become more necessary. Anchoring the invalidation of the NBFDS in science communication teachings would be a strong step for Congress toward a fact-based future.
Conclusion
Thanks to the advent of genetic scissors, bioengineering is now more efficient, precise, and affordable than ever before. This exciting discovery has opened the door to countless other research avenues in a wide variety of fields from medicine to agriculture. Bioengineered crops in particular have incredible potential for food security, climate change, and even developing nations’ health and economies.
And yet, scientists’ failure to communicate the advantages of bioengineered crops has left the lay public and politicians misinformed. Generalized negative perceptions and regurgitation of inaccurate talking points are rampant in public discourse surrounding bioengineered foods, and the U.S. Congress has fallen prey to such misinformation.
By requiring a “bioengineered food” label on packaging, the government is violating the First Amendment rights of commercial speakers. Importantly, the label itself invokes a polarized debate steeped in falsities. Moreover, the label does nothing to inform consumers about any valuable health, safety, or other guidance. In fact, the label is prone to confuse and further mislead consumers. Thus, the NBFDS cannot realistically contribute to a robust marketplace of ideas or further democratic self-governance principles.
The bioengineered food disclosure, as implemented by the NBFDS, is not only unconstitutional, but it also hurts the economy, does not contribute to knowledgeable consumer choice, fuels misinformation and disinformation, and stifles innovation. It is unrealistic to think that humans of this century can live in a world without bioengineered foods to feed the human population, and the erroneous notion that bioengineered foods are alarming enough to deserve their own label should be quashed.
*J.D. Candidate, 2024, University of Colorado Law School. B.S., Genetics; Certificate, Integrated Studies in Science, Engineering, and Society, University of Wisconsin-Madison. Thank you to Professor Helen Norton for sparking my interest in the First Amendment and for graciously offering her time and feedback on this Note. Thanks also to each and every one of my University of Colorado Law Review colleagues who put eyes on this piece, but especially to my very wise and thoughtful editorial team: Caitlin Dacus, Ricardo Zorce, Mykaela Delgado Schluter, Mary Slosson, Xandra Nielsen, and Catherine Kelly. Finally, thank you to my family for their endless love and support in all of my endeavors that led me to this accomplishment.
- Charles Darwin, On the Origin of Species 109 (D. Appleton & Co. 1861). ↑
- Gabriel Rangel, From Corgis to Corn: A Brief Look at the Long History of GMO Technology, SITNBoston (Aug. 9, 2015), https://sitn.hms.harvard.edu/flash/2015/from-corgis-to-corn-a-brief-look-at-the-long-history-of-gmo-technology [https://perma.cc/6SP8-8PTX] (“The earliest evidence of artificial selection of plants dates back to 7800 BCE in archaeological sites found in southwest Asia, where scientists have found domestic varieties of wheat.”). Additionally, Rangel discusses the domesticated dog as potentially the first instance of artificial selection by humans. Id. ↑
- See Why Do Farmers in the U.S. Grow GMO Crops?, FDA, https://www.fda.gov/food/agricultural-biotechnology/why-do-farmers-us-grow-gmo-crops [https://perma.cc/8423-PRHQ] (Feb. 17, 2022). ↑
- See id. ↑
- See Rangel, supra note 2. ↑
- See id. ↑
- David Zilberman, Tim G. Holland & Itai Trilnick, Agricultural GMOs—What We Know and Where Scientists Disagree, 10 Sustainability 1, 3 (2018) [hereinafter Zilberman et al., Agricultural GMOs]. Traditional breeding processes used to rely heavily on what scientists could physically observe about their subjects. In a traditional breeding setting, scientists would attempt to change physical outcomes without controlling for actual changes in genetic expression. Id. In contrast, bioengineering methods specifically target genetic expression in the hopes of achieving physical or performance-based outcomes. Id. ↑
- See Press Release, Royal Swedish Academy of Sciences, The Nobel Prize in Chemistry 2020 (Oct. 7, 2020), https://www.nobelprize.org/uploads/2020/10/press-chemistryprize2020.pdf [https://perma.cc/5BX5-AUVJ]. ↑
- See id. (comparing prior methods to genetic scissors and stating that “it is now possible to change the code of life over the course of a few weeks”). CRISPR is an acronym for “clustered regularly interspaced short palindromic repeats,” while Cas9 refers to a protein that binds to genetic material. Milan Kumar Samanta, Avishek Dey & Srimonta Gayen, CRISPR/Cas9: An Advanced Tool for Editing Plant Genomes, 25 Transgenic Rsch. 561, 564–65 (2016). ↑
- Press Release, supra note 8; Samanta et al., supra note 9, at 564. ↑
- Press Release, supra note 8. ↑
- See Rangel, supra note 2 (citing not only the media, but also government officials and scientists). ↑
- See, e.g., Alessandro Marcon et al., CRISPR in the North American Popular Press, 21 Genetics in Med. 2184, 2188 (2019) (“Compared with the media discourse surrounding other recent biomedical topics, . . . the current media portrayal of CRISPR includes a higher percentage of articles containing risks/concerns.”). ↑
- See infra Sections I.A.2, I.B.2. ↑
- About the Non-GMO Project, Non-GMO Project, https://www.nongmoproject.org/about [https://perma.cc/79B7-4CVX]. GMO stands for “genetically modified organism.” ↑
- See, e.g., NonGMOProject (@NonGMOProject), X (Sept. 29, 2022, 6:05 PM), https://twitter.com/NonGMOProject/status/1575637802066350080 [https://perma.cc/EC22-7SGB] (site previously called “Twitter”) (expressing distrust of bioengineered foods and disagreement with President Biden’s Executive Order). I also encourage the reader to run a web search with “GMO health and environmental risks” in the search bar and assess their top sponsored results. ↑
- See generally Kevin Doxzen & Hope Henderson, Is This Safe? Addressing Societal Concerns About CRISPR-Edited Foods Without Reinforcing GMO Framing, 14 Env’t Commc’n 865 (2020) (calling on science communicators to frame products created with CRISPR-Cas9 genetic scissors differently than products made via traditional breeding methods in the hopes that consumers will support genetic scissors technology); cf. Tamar Haspel, The Public Doesn’t Trust GMOs. Will It Trust CRISPR?, Vox (July 26, 2018, 4:02 PM), https://www.vox.com/science-and-health/2018/7/18/17586198/crispr-food-agriculture-national-academies-science-gmos [https://perma.cc/4ZAH-9FMK] (distinguishing CRISPR-Cas9 from traditional bioengineering in agriculture because of its comparative transparency). ↑
- Advancing Biotechnology and Biomanufacturing Innovation for a Sustainable, Safe, and Secure American Bioeconomy, 87 Fed. Reg. 56849 (Sept. 12, 2022). ↑
- See id. at 56850 (listing eleven subparts as the policy of the Biden administration, relating to research and development, manufacturing and production, environmental sustainability, workforce implications, product outcomes, risk management, policy and standard development, national security, and international cooperation). ↑
- Id. at 56849. ↑
- S. Rep. No. 114-403, at 4 (2016) (“The mandatory disclosure requirement is designed solely to address marketing matters, not based on any concerns with respect to safety of bioengineered foods or ingredients . . . .”). ↑
- Id. ↑
- Food Lobby Spends $101 Million in 2015 to Avert GMO Labeling, Env’t Working Grp. (Feb. 25, 2016), https://www.ewg.org/research/food-lobby-spends-101-million-2015-avert-gmo-labeling [https://perma.cc/DEY8-WHZ8]. ↑
- 7 U.S.C. § 1639b(a)(1) (2016). ↑
- Id. § 1639b(b)(2)(D). ↑
- See 7 C.F.R. §§ 66.100–66.108 (2019). ↑
- See, e.g., Rubin v. Coors Brewing Co., 514 U.S. 476, 481 (1995) (finding without question that information on beer labels constitutes commercial speech); Pearson v. Shalala, 164 F.3d 650, 655 (D.C. Cir. 1999) (declaring that the commercial speech doctrine application to health claim restrictions on dietary supplement labels is “undisputed”); Turtle Island Foods SPC v. Strain, 594 F.Supp.3d 692, 701 (M.D.L., 2022) (finding that the labeling of plant-based meat products invokes commercial speech analysis), rev’d and vacated sub nom. Turtle Island Foods, SPC v. Strain, 65 F.4th 211 (5th Cir. 2023). ↑
- 471 U.S. 626 (1985). ↑
- Id. at 651. The government may have an alternative interest that does not fall under the umbrella of “preventing deception of consumers.” See, e.g., Nat’l Elec. Mfrs. Ass’n v. Sorrell, 272 F.3d 104, 115 n.6 (2d Cir. 2001) (finding a government interest in protecting human health and the environment). This is a point of tension in the test, as discussed infra in Section II.B.2. ↑
- 447 U.S. 557 (1980). ↑
- Id. at 566. ↑
- See Jennifer M. Keighley, Can You Handle the Truth? Compelled Commercial Speech and the First Amendment, 15 J. Const. L. 539, 550 (2012). ↑
- See id. at 552. ↑
- See infra Section I.A.2. ↑
- See Doxzen & Henderson, supra note 17, at 869 (“Establishing CRISPR as a neutral tool with a range of applications gives stakeholders a voice on which applications we should pursue, expectations of regulations, and other common causes of concern.”). ↑
- Genetically modified organisms (GMOs) encompass not just agricultural crops (plants), but also animals and microbes. Genetically Modified Organism (GMO), NIH Nat’l Hum. Genome Rsch. Inst., https://www.genome.gov/genetics-glossary/Genetically-Modified-Organism [https://perma.cc/P6WZ-7R6U] (Dec. 29, 2023). GMOs may also be used for other purposes outside of agriculture, as alluded to previously in this Note. Id.; see also Introduction. The acronym is commonly used in the bioengineered food conversation, as evidenced by a quick web search and as exhibited by the name of “The Non-GMO Project,” an organization that focuses on food labeling. ↑
- Zilberman et al., Agricultural GMOs, supra note 7, at 3. For the publication detailing the discovery itself, see Martin Jinek, Krzysztof Chylinski, Ines Fonfara, Michael Hauer, Jennifer A. Doudna & Emmanuelle Charpentier, A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity, Science (2012). ↑
- Zilberman et al., Agricultural GMOs, supra note 7, at 3. ↑
- See id. ↑
- See id. ↑
- Here, “organic” refers to its core chemical meaning: “relating to, being, or dealt with by a branch of chemistry concerned with the carbon compounds of living beings and most other carbon compounds.” Organic, Merriam-Webster, https://www.merriam-webster.com/dictionary/organic [https://perma.cc/BX8Q-37PK]. ↑
- Another helpful analogy may be the use of different beads to make a bracelet, where certain combinations can create various patterns such as rainbows, alternating colors, snaked patterns, and more. ↑
- Johan Jarnestad, The CRISPR/Cas9 Genetic Scissors, Royal Swedish Acad. of Scis. (2020), https://www.nobelprize.org/uploads/2020/10/chemistry-2020-figure3-en.pdf [https://perma.cc/WU7K-9SSH]. ↑
- Deoxyribonucleic Acid (DNA) Fact Sheet, Nat’l Hum. Genome Rsch. Inst., https://www.genome.gov/about-genomics/fact-sheets/Deoxyribonucleic-Acid-Fact-Sheet [https://perma.cc/XC9U-78AY] (Aug. 24, 2020). For readers interested in the full chemical names of these building blocks, they are adenine, cytosine, thymine, and guanine. Id. ↑
- Songwriting 101: Learn Common Song Structures, MasterClass, https://www.masterclass.com/articles/songwriting-101-learn-common-song-structures [https://perma.cc/ZDX8-ZPV4] (Aug. 10, 2021). ↑
- See Carl Zimmer, CRISPR, 10 Years On: Learning to Rewrite the Code of Life, N.Y. Times (June 30, 2022), https://www.nytimes.com/2022/06/27/science/crispr-gene-editing-10-years.html [https://perma.cc/RW6J-2A6A] (“In just a decade, CRISPR has become one of the most celebrated inventions in modern biology.”). Interestingly, the discovery was not immediately recognized by popular media. Id. ↑
- Press Release, supra note 8. ↑
- See Matthew Niederhuber, Insecticidal Plants: The Tech and Safety of GM Bt Crops, SITNBoston (Aug. 10, 2015), https://sitn.hms.harvard.edu/flash/2015/insecticidal-plants [https://perma.cc/28ZH-SAPZ]; Zilberman et al., Agricultural GMOs, supra note 7, at 8. Producers may also use other strategies such as increased shelf life or enhanced flavor. See, e.g., Michael J. Haas, New Gene Could Help Improve Tomato Flavor and Shelf Life, Cornell Chron. (Oct. 19, 2021), https://news.cornell.edu/stories/2021/10/new-gene-could-help-improve-tomato-flavor-and-shelf-life [https://perma.cc/9A4J-657N]. ↑
- See Haspel, supra note 17; Zilberman et al., Agricultural GMOs, supra note 7, at 3, 12; see, e.g., GMO & Biotechnology, Bayer Glob., https://www.bayer.com/en/agriculture/gmo-biotechnology [https://perma.cc/9QRX-2R4E] (March 30, 2023) (stating that the average cost to take a bioengineered seed to market is $130 million over thirteen years). ↑
- See Chemical Safety: Pesticides, WHO (Oct. 26, 2020), https://www.who.int/news-room/questions-and-answers/item/chemical-safety-pesticides [https://perma.cc/D4LN-AUDA]. ↑
- See Nat’l Rsch. Council, The Impact of Genetically Engineered Crops on Farm Sustainability in the United States 62, 68 (2010); Zilberman et al., Agricultural GMOs, supra note 7, at 5. ↑
- Niederhuber, supra note 48. ↑
- Id. ↑
- Id. ↑
- See id.; Zilberman et al., Agricultural GMOs, supra note 7, at 4. ↑
- See Nat’l Rsch. Council, supra note 51, at 93; Zilberman et al., Agricultural GMOs, supra note 7, at 6. ↑
- Zilberman et al., Agricultural GMOs, supra note 7, at 4, 15. ↑
- Id. at 15. The United Nations Cartagena Protocol is the main reason for widespread delays; it binds the European Union to a “zealously overcautious” approach with respect to bioengineered food regulation. Ed Regis, Opinion: Golden Rice Could Save Children. Until Now, Governments Have Barred It., Wash. Post (Nov. 11, 2019, 5:46 PM), https://www.washingtonpost.com/opinions/2019/11/11/golden-rice-long-an-anti-gmo-target-may-finally-get-chance-help-children [https://perma.cc/AUF5-2SBF]. ↑
- David Zilberman, Scott Kaplan & Justus Wesseler, The Loss from Underutilizing GM Technologies, 18 AgBioForum 312, 315 (2015). ↑
- Id. ↑
- Philippines Becomes First Country to Approve Nutrient-Enriched “Golden Rice” for Planting, Int’l Rice Rsch. Inst. (July 23, 2021), https://www.irri.org/news-and-events/news/philippines-becomes-first-country-approve-nutrient-enriched-golden-rice [https://perma.cc/Q6U4-W32R]. ↑
- See generally Aaron M. Shew et al., CRISPR Versus GMOs: Public Acceptance and Valuation, 19 Glob. Food Sec. 71 (2018). ↑
- Cross-pollination concerns also come up in the discussion of allergenicity of bioengineered crops. See infra Section I.A.2. ↑
- Heather Landry, Challenging Evolution: How GMOs Can Influence Genetic Diversity, SITNBoston (Aug. 10, 2015), https://sitn.hms.harvard.edu/flash/2015/challenging-evolution-how-gmos-can-influence-genetic-diversity [https://perma.cc/CSA2-QWD8]; Zilberman et al., Agricultural GMOs, supra note 7, at 10 (“Experience with soy has in fact shown that much of varietal diversity has been maintained.”) (citation omitted). ↑
- See, e.g., Sara Velardi & Theresa Selfa, Framing Local: An Analysis of Framing Strategies for Genetically Modified Organism (GMO) Labeling Initiatives in the Northeastern U.S., 45 Agroecology & Sustainable Food Sys. 366, 380 (2021). ↑
- Landry, supra note 64. ↑
- Zilberman et al., Agricultural GMOs, supra note 7, at 6. ↑
- Id. at 10. ↑
- Id. at 11 (also referring to the American chestnut and the American elm as species negatively affected by fungi, which geneticists are working diligently to reestablish in their home ranges). ↑
- See id. at 10; Emma Kovak, Dan Blaustein-Rejto & Matin Qaim, Genetically Modified Crops Support Climate Change Mitigation, 27 Trends in Plant Sci. 627, 627 (2022) (finding that growing bioengineered crops in the European Union could reduce greenhouse gas emissions by 33 million tons of carbon dioxide equivalents per year, which is equivalent to 7.5 percent of total EU agricultural emissions in 2017). ↑
- See Climate Change and Agriculture: A Perfect Storm in Farm Country, Union of Concerned Scientists (Mar. 20, 2019), https://www.ucsusa.org/resources/climate-change-and-agriculture [https://perma.cc/TDJ7-W7JY]. ↑
- Kovak et al., supra note 70, at 627. ↑
- Id. ↑
- Id. (citations omitted). Tillage is “turning the soil to control for weeds and pests and to prepare for seeding . . . .” Soil Tillage and Crop Rotation, USDA, https://www.ers.usda.gov/topics/farm-practices-management/crop-livestock-practices/soil-tillage-and-crop-rotation [https://perma.cc/TT3K-P4AM]. ↑
- What Is Carbon Sequestration?, U.S. Geological Surv., https://www.usgs.gov/faqs/what-carbon-sequestration [https://perma.cc/X5CR-MND8]. ↑
- Zilberman et al., Agricultural GMOs, supra note 7, at 10. ↑
- Nat’l Rsch. Council, supra note 51, at 67. ↑
- Zilberman et al., Agricultural GMOs, supra note 7, at 3. ↑
- EPA, Climate Impacts on Agriculture and Food Supply, City of Chi., https://climatechange.chicago.gov/climate-impacts/climate-impacts-agriculture-and-food-supply [https://perma.cc/PD53-HDFW]. ↑
- Zilberman et al., Agricultural GMOs, supra note 7, at 5. ↑
- See L. Val Giddings, Robert D. Atkinson & J. John Wu, Info. Tech. & Innovation Found., Suppressing Growth: How GMO Opposition Hurts Developing Nations 4 (2016). ↑
- Id. at 5–6 (labeling this phenomenon “green imperialism”). ↑
- Id. at 1. ↑
- Zilberman et al., Agricultural GMOs, supra note 7, at 12. ↑
- Jane E. Brody, Are G.M.O. Foods Safe?, N.Y. Times (Apr. 23, 2018), https://www.nytimes.com/2018/04/23/well/eat/are-gmo-foods-safe.html [https://perma.cc/Z9XM-P9ML]. ↑
- See supra Section I.A.1. ↑
- See Patricia Cohen, Roundup Weedkiller Is Blamed for Cancers, but Farmers Say It’s Not Going Away, N.Y. Times (Sept. 20, 2019), https://www.nytimes.com/2019/09/20/business/bayer-roundup.html [https://perma.cc/D87K-XT9K]. ↑
- Jordan Wilkerson, Why Roundup Ready Crops Have Lost their Allure, SITNBoston (Aug. 10, 2015), https://sitn.hms.harvard.edu/flash/2015/roundup-ready-crops [https://perma.cc/5ZZR-GYBQ]. ↑
- See id.; Zilberman et al., Agricultural GMOs, supra note 7, at 5. When herbicide is used properly and in accordance with federal guidelines, herbicide-resistant bioengineered crops have very small trace amounts of herbicide remaining on them from cultivation. Glyphosate, EPA, https://www.epa.gov/ingredients-used-pesticide-products/glyphosate [https://perma.cc/YR7J-LYLZ] (Sept. 11, 2023). These trace amounts do not negatively impact human health. Id. ↑
- How GMO Crops Impact Our World, FDA, https://www.fda.gov/food/agricultural-biotechnology/how-gmo-crops-impact-our-world [https://perma.cc/KR3H-RM37] (Apr. 19, 2023). ↑
- See David Barboza, The Power of Roundup; A Weed Killer Is a Block for Monsanto to Build on, N.Y. Times (Aug. 2, 2001), https://www.nytimes.com/2001/08/02/business/the-power-of-roundup-a-weed-killer-is-a-block-for-monsanto-to-build-on.html [https://perma.cc/Q38H-BTNZ]. This marketing strategy sparked litigation against Monsanto: “The DuPont Company has filed two lawsuits in federal courts accusing Monsanto of violating antitrust laws by linking the sale of Roundup and Roundup Ready crops and by using incentives and requirements to lock out rivals.” Id. ↑
- Wilkerson, supra note 88. Farmers’ high herbicide uses could be due to plain carelessness but more likely stem from the stress that farmers often face with respect to their yields and lifestyle. See Debbie Weingarten, Why Are America’s Farmers Killing Themselves?, Guardian (Dec. 11, 2018, 12:51), https://www.theguardian.com/us-news/2017/dec/06/why-are-americas-farmers-killing-themselves-in-record-numbers [https://perma.cc/6CDP-X64T]. ↑
- Wilkerson, supra note 88. ↑
- Id.; Glyphosate, supra note 89. ↑
- Johnathan Hettinger, ‘Buy It or Else’: Inside Monsanto and BASF’s Moves to Force Dicamba on Farmers, Investigate Midwest (Dec. 4, 2020), https://investigatemidwest.org/2020/12/04/buy-it-or-else-inside-monsanto-and-basfs-moves-to-force-dicamba-on-farmers [https://perma.cc/5RAW-KRGJ]. ↑
- Frank Morris, Monsanto GMO Ignites Big Seed War, NPR (Jan. 12, 2010, 3:00 PM), https://www.npr.org/templates/story/story.php?storyId=122498255 [https://perma.cc/2V5T-C7PF] (quoting a Kansas farmer: “There’s nothing like Roundup. A monkey could farm with it.”). ↑
- See Hettinger, supra note 95. ↑
- See id. (detailing the story of Illinois farmer Will Glazik). ↑
- See Cohen, supra note 87. ↑
- Mari Gaines, Roundup Lawsuit Update February 2024, Forbes Advisor, https://www.forbes.com/advisor/legal/product-liability/roundup-lawsuit-update [https://perma.cc/F7HA-ESF3] (Feb. 2, 2024, 9:43 AM). The article also details that “Monsanto has settled over 100,000 Roundup lawsuits, paying out about $11 billion as of May 2022.” Id. ↑
- See Cohen, supra note 87. ↑
- See id. ↑
- Charles Xu, Nothing to Sneeze at: The Allergenicity of GMOs, SITNBoston (Aug. 10, 2015), https://sitn.hms.harvard.edu/flash/2015/allergies-and-gmos [https://perma.cc/YSC6-7RR3]. ↑
- Id. ↑
- See discussion of insecticidal bioengineered crops supra Section I.A.1. ↑
- Xu, supra note 103. ↑
- Id. ↑
- CDC, Investigation of Human Health Effects Associated with Potential Exposure to Genetically Modified Corn 3 (2011), https://www.cdc.gov/nceh/ehhe/cry9creport/pdfs/cry9creport.pdf [https://perma.cc/GLJ8-WGG7]. ↑
- Id. at 10. ↑
- Id. ↑
- Xu, supra note 103. ↑
- See Peter Thomison & Allen Geyer, Managing “Pollen Drift” to Minimize Contamination of Non-GMO Corn, Ohioline (Mar. 15, 2016), https://ohioline.osu.edu/factsheet/agf-153 [https://perma.cc/5KMC-NMYJ]. ↑
- Id. ↑
- Id. ↑
- USDA, Minimum Separation Distances to be Used for Confined Field Tests of Certain Genetically Engineered Plants (2013), https://www.aphis.usda.gov/biotechnology/downloads/sep_dist_table_0813.pdf [https://perma.cc/SYZ7-DRFA] (offering guidance for an Animal and Plant Health Inspection Service regulation no longer in force). ↑
- See Julie K. Smith, The National Bioengineered Food Disclosure Standard: A Statute in Need of a Do-Over, 27 Kan. J.L. & Pub. Pol’y 1, 22 (2017) (“Genetic engineering does not inherently give rise to more harmful products than other methods.”). ↑
- How GMOs Are Regulated in the United States, FDA, https://www.fda.gov/food/agricultural-biotechnology/how-gmos-are-regulated-united-states [https://perma.cc/ZUX6-SRS8] (Apr. 19, 2023); Coordinated Framework for Regulation of Biotechnology, 51 Fed. Reg. 23302 (June 26, 1986). ↑
- Baruch Fischhoff & Dietram A. Scheufele, The Science of Science Communication, 110 PNAS 14031, 14031 (2013). ↑
- Id. ↑
- See id. ↑
- See, e.g., Tucker Carlson, The Coronavirus Pandemic Is a Global Fraud Perpetrated by China, Abetted by the Powerful, Fox News (Dec. 2, 2020, 10:58 PM), https://www.foxnews.com/opinion/tucker-carlson-on-chinas-global-fraud-coronavirus [https://perma.cc/7AR2-45DN]; Natalie O’Neill, You’re No Safer From COVID-19 Social Distancing at 6 or 60 Feet, Study Says, N.Y. Post (Apr. 26, 2021, 12:08 PM), https://nypost.com/2021/04/25/youre-no-safer-from-covid-social-distancing-at-6-or-60-feet-study [https://perma.cc/E2PP-7FUB]; Charles Kim, Sen. Marshall to Newsmax: Studies Say Masks Don’t Protect Against COVID, Newsmax (Aug. 31, 2021, 9:40 PM), https://www.newsmax.com/newsmax-tv/marshall-covid-masks-mandates/2021/08/31/id/1034554 [https://perma.cc/824R-X492]; Kristine Varkony, Anti-Vaxxer Tells Ohio Lawmakers COVID-19 Vaccine Can Leave People Magnetized, Interfaced with 5G Towers, NBC4i.com (June 8, 2021, 5:12 PM), https://www.nbc4i.com/news/local-news/anti-vaxxer-tells-ohio-lawmakers-covid-19-vaccine-can-leave-people-magnetized-interfaced-with-5g-towers [https://perma.cc/W5CL-T57N]. ↑
- A Martínez & Allison Aubrey, How Vaccine Misinformation Made the COVID-19 Death Toll Worse, NPR (May 16, 2022, 5:07 AM), https://www.npr.org/2022/05/16/1099070400/how-vaccine-misinformation-made-the-covid-19-death-toll-worse [https://perma.cc/QJ2G-77MU]. ↑
- Marko Ahteensuu, Assumptions of the Deficit Model Type of Thinking: Ignorance, Attitudes, and Science Communication in the Debate on Genetic Engineering in Agriculture, 25 J. Agric. & Env’t Ethics 295, 296 (2012). ↑
- Id. ↑
- Kathleen M. Rose et al., Distinguishing Scientific Knowledge: The Impact of Different Measures of Knowledge on Genetically Modified Food Attitudes, 28 Pub. Understanding Sci. 449, 450 (2019) [hereinafter Rose et al., Distinguishing Scientific Knowledge]. ↑
- Ahteensuu, supra note 123, at 309. ↑
- The Public, the Media and Agricultural Biotechnology 232 (Dominique Brossard, James Shanahan & T. Clint Nesbitt eds., 2007). ↑
- Id. at 233. ↑
- See Sara Velardi & Theresa Selfa, Framing Local: An Analysis of Framing Strategies for Genetically Modified Organism (GMO) Labeling Initiatives in the Northeastern U.S, 45 Agroecology & Sustainable Food Sys. 366, 369 (2021). ↑
- Id. ↑
- See, e.g., Amanda Barrell, Genetically Modified Food: What Are the Pros and Cons?, MedicalNewsToday, https://www.medicalnewstoday.com/articles [https://perma.cc/DCM4-27DQ] (Jan. 12, 2023) (framing “pros and cons” with equal value and stating that “[g]enetically engineering foods is a relatively new practice, which means the long-term effects on safety are not yet clear”); Kelsey Blackwell, How Bad Are GMOs For Your Health? Here’s the GMO Health Science, Made in Nature (Nov. 2, 2017), https://www.madeinnature.com/blogs/snack-life/how-bad-are-gmos-for-your-health-here-s-the-gmo-health-science [https://perma.cc/CX58-WQEQ] (conflating correlation with causation with respect to bioengineered foods and allergy rates in children and suggesting bioengineered foods also cause infertility). ↑
- Rose et al., Distinguishing Scientific Knowledge, supra note 125, at 451. ↑
- Id. ↑
- Kathleen M. Rose, Dominique Brossard & Dietram A. Scheufele, Of Society, Nature, and Health: How Perceptions of Specific Risks and Benefits of Genetically Engineered Foods Shape Public Rejection, 14 Env’t Commc’n 1017, 1027 (2020) [hereinafter Rose et al., Of Society, Nature, and Health]. ↑
- Id. ↑
- Id. ↑
- Rose et al., Distinguishing Scientific Knowledge, supra note 125, at 457. ↑
- Id. at 462; see also Rose et al., Of Society, Nature and Health, supra note 134 (“[T]hose who pay more attention to GMO-specific news rate the importance of GMO-free foods more highly.”). ↑
- See, e.g., Martin Müller et al., Assessing Public Opinion on CRISPR-Cas9: Combining Crowdsourcing and Deep Learning, 22 J. Med. Internet Rsch. no. 8, 2020, at 9. (examining CRISPR sentiment on Twitter and finding a recent “series of strong negative dips, pointing to a more critical view”). ↑
- Velardi & Selfa, supra note 65, at 366. ↑
- Id. at 367, 374 (listing California, Washington, Oregon, Colorado, Massachusetts, and New York as states where bioengineered food labeling measures failed). ↑
- Id. at 373. ↑
- Id. at 367. ↑
- Id. at 373. ↑
- Id. at 383. ↑
- Id. at 368. ↑
- Id. at 370. ↑
- Id. at 368. Merriam-Webster defines “agroecology” as “an ecological approach to agriculture that views agricultural areas as ecosystems and is concerned with the ecological impact of agricultural practices.” Agroecology, Merriam-Webster, https://www.merriam-webster.com/dictionary/agroecological [https://perma.cc/N4P5-SA6G]. ↑
- Press Release, USDA, Establishing the National Bioengineered Food Disclosure Standard (Dec. 20, 2018), https://www.usda.gov/media/press-releases/2018/12/20/establishing-national-bioengineered-food-disclosure-standard [https://perma.cc/3TSA-LXMR] (quoting U.S. Secretary of Agriculture Sonny Purdue: “The Standard also avoids a patchwork state-by-state system that could be confusing to consumers.”). ↑
- S. Rep. No. 114-403, at 6 (2016). ↑
- 7 U.S.C. § 1639b(e). ↑
- 7 C.F.R. §§ 66.1, 66.100–66.108. ↑
- S. Rep. No. 114-403, at 4 (2016). ↑
- 622 F.3d 628, 641 (6th Cir. 2010). ↑
- See, e.g., Newly Finalized Federal Regulations on GMOs Are a “Free for All” for Chemical Corporations, Ctr. for Food Safety (May 18, 2020), https://www.centerforfoodsafety.org/press-releases/6014/newly-finalized-federal-regulations-on-gmos-are-a-free-for-all-for-chemical-corporations [https://perma.cc/24YP-3PAX] (depicting a picture of a syringe going into a corncob and threatening that bioengineered crops are “no longer subject to agency oversight or evaluation of harms”). ↑
- Glenn G. Lammi, Crusade or Charade: What’s Really Motivating Efforts to Mandate GMO-Labeling?, Forbes (Aug. 25, 2016, 12:08 PM), https://www.forbes.com/sites/wlf/2016/08/25/crusade-or-charade-whats-really-motivating-efforts-to-mandate-gmo-labeling [https://perma.cc/ZU7R-S8P8]. ↑
- Compare id., with supra Section I.A.1. ↑
- S. Rep. No. 114-403, at 4 (2016). ↑
- IRT’s Mission Statement, IRT, https://responsibletechnology.org/mission-statement [https://perma.cc/SY6Z-ZYYZ]. ↑
- Lammi, supra note 156. ↑
- About OCA, Organic Consumers Ass’n, https://organicconsumers.org/about-oca [https://perma.cc/84JV-AVNB]. ↑
- Lammi, supra note 156. ↑
- Id. ↑
- Id. ↑
- Id. ↑
- See Schenck v. United States., 249 U.S. 47 (1919) (specifically, the Court first directly considered the freedom of expression in its ruling on the 1917 Espionage Act); see also First Amendment Timeline, Free Speech Ctr., https://www.mtsu.edu/first-amendment/page/first-amendment-timeline [https://perma.cc/4KW9-J87F]. ↑
- 316 U.S. 52 (1942). ↑
- Id. at 53. ↑
- Id. at 54. ↑
- Id. ↑
- 425 U.S. 748, 770 (1976). ↑
- Id. at 749–50. ↑
- Id. at 763. ↑
- Id. at 770. ↑
- 447 U.S. 557 (1980). ↑
- Id. at 561–62. ↑
- Id. at 561 (citing Virginia State Board of Pharmacy, 425 U.S. at 762). ↑
- Id. at 561–62. ↑
- Keighley, supra note 32, at 550. ↑
- See id. at 554; Zauderer v. Off. of Disciplinary Couns., 471 U.S. 626, 651 (1985) (“[T]he extension of First Amendment protection to commercial speech is justified principally by the value to consumers of the information such speech provides . . . .”). ↑
- Cent. Hudson, 447 U.S. at 563 (citing Friedman v. Rogers, 440 U.S. 1, 13, 15–16, (1979); Ohralik v. Ohio State Bar Ass’n, 436 U.S. 447, 464–65 (1978); Pittsburgh Press Co. v. Pittsburgh Comm’n on Hum. Rels., 413 U.S. 376, 388 (1973)). ↑
- See supra note 27. ↑
- The Court considered in Nike, Inc. v. Kasky whether speech by a commercial actor, to a commercial audience, with both commercial and noncommercial elements qualified as commercial speech. See 539 U.S. 654, 657, 660 (2003) (J. Stevens, concurring). Here, in response to public outrage at its problematic labor practices, Nike launched a public relations and advertising campaign to address its bad press and bolster its brand. Id. at 656. While the Court ultimately decided not to take the case, citing unresolved issues of fact, id. at 664–65, Justice Stevens, concurring with Justices Ginsberg and Souter, voiced that this situation presented unique questions with respect to commercial speech regulation. Id. at 663. In possibly the most difficult case of identifying commercial speech in modern times, Justice Stevens acknowledged certain factors of importance such as the intent to generate sales, directness of the communication between the commercial actor and the consumer, as well as the potential chilling effect on participation in important public debates. Id. at 663–64. Writing for himself and Justice O’Connor, Justice Breyer dissented, ultimately wishing the Court would have decided this issue. See id. at 684 (J. Breyer, dissenting). He offered factors of his own such as whether the speech appeared in a traditional advertising format, whether it proposed a commercial transaction, and the relevant regulatory context in which the speech was expressed. Id. at 677–78. Because this case posed a unique set of circumstances from which to derive commercial speech, it offers guidance on the outer limits of such speech. Still, no majority has commanded an opinion to solidify these factors. ↑
- While the doctrine is settled today, the Court still struggled to come to terms with this test’s authority. See Robert Post, Compelled Commercial Speech, 117 W. Va. L. Rev. 867, 881 (2015). ↑
- Cent. Hudson, 447 U.S. at 566. ↑
- See Matal v. Tam, 582 U.S. 218, 244–45 (2017). ↑
- See Centro de la Comunidad Hispana de Locust Valley v. Town of Oyster Bay, 868 F.3d 104, 112 (2d Cir. 2017). ↑
- See Recht v. Morrisey, 32 F.4th 398, 405, 408 (4th Cir.), cert. denied, 143 S. Ct. 527 (2022). ↑
- Zauderer v. Off. of Disciplinary Couns., 471 U.S. 626, 651 (1985). ↑
- See Nat’l Elec. Mfrs. Ass’n v. Sorrell, 272 F.3d 104, 115 (2d Cir. 2001). ↑
- See id. at 116. ↑
- Zauderer, 471 U.S. at 651. ↑
- Id. ↑
- Id. But see note 29; infra Section II.B.2. ↑
- Id. ↑
- See Nigel Barrella, First Amendment Limits on Compulsory Labeling, 71 Food & Drug L.J. 519, 539 (2016); Note, Repackaging Zauderer, 130 Harv. L. Rev. 972, 972 (2017) (describing Zauderer as an exception to the Central Hudson test). ↑
- Id. at 633. ↑
- See id. at 652–53. ↑
- Milavetz, Gallop & Milavetz, P.A. v. United States, 559 U.S. 229, 249 (2010). ↑
- 272 F.3d 104, 107 (2d Cir. 2001). ↑
- See id. at 113–14. ↑
- See id. at 115–16. ↑
- Zauderer, 471 U.S. at 651; see discussion infra Section II.B.2 (analyzing the confusion of this language in Zauderer). ↑
- Sorrell, 272 F.3d at 115. ↑
- Id. at 114. ↑
- Id. ↑
- 92 F.3d 67 (2d Cir. 1996). ↑
- Id. at 69. ↑
- Id. at 73–74. ↑
- Id. ↑
- Id. ↑
- See id. ↑
- Int’l Dairy Foods Ass’n v. Boggs, 622 F.3d 628 (6th Cir. 2010). ↑
- Id. at 633. ↑
- Id. at 634. ↑
- Id. at 642. ↑
- Id.; see also Disc. Tobacco City & Lottery, Inc. v. United States, 674 F.3d 509, 523–24 (6th Cir. 2012) (“[T]he government has more leeway to regulate potentially misleading commercial speech” than nonmisleading speech.). ↑
- Boggs, 622 F.3d at 636. ↑
- See id. at 642. ↑
- Zauderer v. Off. of Disciplinary Couns., 471 U.S. 626, 651 (1985). ↑
- See Disc. Tobacco City & Lottery, 674 F.3d at 569. (“[W]hether a disclosure is scrutinized under Zauderer turns on whether the disclosure conveys factual information or an opinion, not on whether the disclosure emotionally affects its audience or incites controversy.”); see also Lauren Fowler, The “Uncontroversial” Controversy in Compelled Commercial Disclosures, 87 Fordham L. Rev. 1651, 1675 (2019). ↑
- See Disc. Tobacco City & Lottery, 674 F.3d at 569. ↑
- Nat’l Inst. of Fam. & Life Advocs. v. Becerra, 138 S. Ct. 2361, 2368 (2018). ↑
- Id. at 2372 (rejecting the Zauderer test as applied to abortion-related disclosures). ↑
- CTIA—The Wireless Ass’n v. City of Berkeley, 928 F.3d 832, 848 (9th Cir. 2019). ↑
- CTIA—The Wireless Ass’n v. City of Berkeley, 854 F.3d 1105, 1117 (9th Cir. 2017), vacated, 138 S. Ct. 2708 (2018). ↑
- It is important to note that some scholars question whether the NIFLA decision is based securely in, and can be relied on as, compelled commercial speech doctrine. See, e.g., Erwin Chemerinsky & Michele Goodwin, Constitutional Gerrymandering Against Abortion Rights: NIFLA v. Becerra, 94 N.Y.U. L. Rev. 61, 66 (2019) (describing NIFLA as “primarily about five conservative Justices’ hostility to abortion rights”). ↑
- Zauderer v. Off. of Disciplinary Couns., 471 U.S. 626, 651 (1985). ↑
- See Post, supra note 184, at 882. ↑
- See, e.g., Nat’l Elec. Mfrs. Ass’n v. Sorrell, 272 F.3d 104, 115 n.6 (2d Cir. 2001) (finding a government interest in protecting human health and the environment); N.Y. State Rest. Ass’n v. N.Y.C. Bd. of Health, 556 F.3d 114, 133 (2d Cir. 2009) (relying on and agreeing with Sorrell); CTIA, 928 F.3d at 844 (finding a government interest in furthering public health and safety); Am. Meat Inst. v. U.S. Dep’t of Agric., 760 F.3d 18, 23 (D.C. Cir. 2014) (positing that adequate government interests may come in different forms). ↑
- See Barrella, supra note 196, at 569 (citing Nat’l Ass’n of Mfrs. v. S.E.C., 800 F.3d 518, 524 (D.C. Cir. 2015); Evergreen Ass’n, Inc. v. City of N.Y., 740 F.3d 233, 245 (2d Cir. 2014)). ↑
- See Barrella, supra note 196, at 539 (citing R.J. Reynolds Tobacco Co. v. FDA, 696 F.3d 1205, 1217 (D.C. Cir. 2012)); see also Note, Repackaging Zauderer, supra note 196, at 972 (describing Zauderer as an exception to the Central Hudson test). ↑
- Zauderer, 471 U.S. at 651. ↑
- See Barrella, supra note 196, at 539; Note, Repackaging Zauderer, supra note 232, at 972. ↑
- Id. ↑
- 529 F. Supp. 3d 1099, 1120 (E.D. Cal. 2021), aff’d sub nom. Cal. Chamber of Com. v. Council for Educ. & Rsch. on Toxics, 29 F.4th 468 (9th Cir. 2022). ↑
- Acrylamide Questions and Answers, FDA (Feb. 25, 2022), https://www.fda.gov/food/process-contaminants-food/acrylamide-questions-and-answers [https://perma.cc/3GKP-AP9F] (defining acrylamide as “a chemical that can form in some foods during high-temperature cooking processes, such as frying, roasting, and baking). ↑
- See Becerra, 529 F. Supp. 3d at 1117. ↑
- Id. ↑
- Id. ↑
- Id. at 1118. ↑
- See supra Section II.B.2. ↑
- Becerra, 529 F. Supp. 3d at 1104–05. ↑
- Cal. Chamber of Com. v. Council for Educ. & Rsch. on Toxics, 29 F.4th 468, 478–79 (9th Cir. 2022). ↑
- See Nat’l Inst. of Fam. & Life Advocs. v. Becerra, 138 S. Ct. 2361, 2372 (2018). ↑
- See id.; supra Section II.B.2. ↑
- Nat’l Inst. of Fam. & Life Advocs., 138 S. Ct. at 2372. ↑
- Id. ↑
- Id. ↑
- Nat’l Elec. Mfrs. Ass’n v. Sorrell, 272 F.3d 104, 114 (2d Cir. 2001). ↑
- CTIA—The Wireless Ass’n v. City of Berkeley, 928 F.3d 832, 845 (9th Cir. 2019). ↑
- Int’l Dairy Foods Ass’n v. Amestoy, 92 F.3d 67, 76 (2d Cir. 1996) (Leval, J., dissenting). ↑
- See supra Section I.B.2. ↑
- See supra Section I.B.2. ↑
- Food Lobby Spends $101 Million in 2015 to Avert GMO Labeling, supra note 23. ↑
- See Cal. Chamber of Com. v. Becerra, 529 F. Supp. 3d 1099, 1117 (E.D. Cal. 2021), aff’d sub nom. Cal. Chamber of Com. v. Council for Educ. & Rsch. on Toxics, 29 F.4th 468 (9th Cir. 2022). ↑
- Compare id., with supra Section I.A. ↑
- See Becerra, 529 F. Supp. 3d at 1120. ↑
- See supra Section I.B.2. ↑
- See supra Section II.B.2. ↑
- CTIA—The Wireless Ass’n v. City of Berkeley, 928 F.3d 832, 844 (9th Cir. 2019). ↑
- Milavetz, Gallop & Milavetz, P.A. v. United States, 559 U.S. 229, 251 (2010) (quoting Zauderer v. Off. of Disciplinary Couns., 471 U.S. 626, 652 (1985)). ↑
- Nat’l Inst. of Fam. & Life Advocs. v. Becerra, 138 S. Ct. 2361, 2367 (2018) (quoting Ibanez v. Fla. Dep’t of Bus. & Prof’l. Regul., 512 U.S. 136, 146 (1994)). ↑
- Video Software Dealers Ass’n v. Schwarzenegger, 556 F.3d 950, 966 (9th Cir. 2009), aff’d sub nom. Brown v. Ent. Merch. Ass’n, 564 U.S. 786 (2011). The Ninth Circuit ran into this issue when deciding whether to strike down a disclosure that applied to “violent” video games. Id. ↑
- Int’l Dairy Foods Ass’n v. Amestoy, 92 F.3d 67, 73 (2d Cir. 1996). ↑
- Int’l Dairy Foods Ass’n v. Boggs, 622 F.3d 628, 642 (6th Cir. 2010). ↑
- Post, supra note 184, at 915. ↑
- Id. ↑
- See supra Section I.B.1 (describing framing as a powerful tool for shaping public perception). ↑
- See supra Section II.B.2. ↑
- CTIA—The Wireless Ass’n v. City of Berkeley, 928 F.3d 832, 844 (9th Cir. 2019) (quoting Nat’l Elec. Mfrs. Ass’n v. Sorrell, 272 F.3d 104, 115 n.6 (2d Cir. 2001)); Am. Beverage Ass’n v. City & Cnty. of S.F., 916 F.3d 749, 756 (9th Cir. 2019) (en banc). ↑
- Sorrell, 272 F.3d at 115. ↑
- Id. ↑
- Id. at 114. ↑
- Id. ↑
- Int’l Dairy Foods Ass’n v. Amestoy 92 F.3d 67, 73 (2d Cir. 1996). ↑
- Id. ↑
- Id. at 74. ↑
- See Andrew Sorensen, Genetically Modified Food Opponents Know Less Than They Think, Research Finds, CU Boulder Today (Jan. 11, 2019), https://www.colorado.edu/today/2019/01/11/genetically-modified-food-opponents-know-less-they-think-research-finds [https://perma.cc/88KJ-QDGE]. ↑
- Keighley, supra note 32, at 551. ↑
- See Mercury Emissions: The Global Context, EPA, https://www.epa.gov/international-cooperation/mercury-emissions-global-context [https://perma.cc/87KH-6KLE] (Apr. 25, 2023). ↑
- Daniel Norero, GMO 25-Year Safety Endorsement: 280 Science Institutions, More Than 3,000 Studies, Genetic Literacy Project (Jan. 21, 2022), https://geneticliteracyproject.org/2022/01/21/gmo-20-year-safety-endorsement-280-science-institutions-more-3000-studies [https://perma.cc/5AB5-K5NP]; see Brody, supra note 85. ↑
- See Lammi, supra note 156. ↑
- Zauderer v. Off. of Disciplinary Couns., 471 U.S. 626, 651 (1985). ↑
- Am. Beverage Ass’n v. City & Cnty. of S.F., 916 F.3d 749, 753, 757 (9th Cir. 2019). ↑
- Id. ↑
- Consol. Cigar Corp. v. Reilly, 218 F.3d 30, 54–55 (1st Cir. 2000), overruled on other grounds by Lorillard Tobacco Co. v. Reilly, 533 U.S. 525 (2001). ↑
- 7 C.F.R. § 66.100(c). ↑
- Id. § 66.118. ↑
- See supra Section I.B. ↑
- While commercial speakers may be better equipped to communicate with a public audience, they may still run into barriers communicating bioengineering technology and how it relates to their products. Additionally, commercial speakers may struggle with issues of trustworthiness in such communications. After all, the main goals and expertise of commercial speakers lie in business. ↑
- Cent. Hudson Gas & Elec. Corp. v. Pub. Serv. Comm’n of New York, 447 U.S. 557, 566 (1980). ↑
- See supra Section III.B. ↑
- Cent. Hudson, 447 U.S. at 566. ↑
- See S. Rep. No. 114-403, at 4 (2016). ↑
- See Safelite Grp., Inc. v. Jepsen, 764 F.3d 258, 265 (2d Cir. 2014) (holding that a restriction on commercial speech failed the third Central Hudson prong, “even if we were to acknowledge the government’s substantial interest in consumer choice”). ↑
- Cent. Hudson, 447 U.S. at 566. ↑
- Id. ↑
- See Bianca Ramirez, The Ineffectiveness of the Current Regulatory System: How the EPA Has Failed to Protect Farmworkers from Pesticide Exposure, 28 Tex. Hisp. J.L. & Pol’y 49, 52–54, 69 (2022). ↑
- See supra Section I.A.2. ↑
- See Jeff Daniels, Elizabeth Warren Slams Big Agriculture, Unveils Plan for Reversing ‘Anti-Competitive Mergers’, CNBC (Mar. 27, 2019, 6:03 PM), https://www.cnbc.com/2019/03/27/elizabeth-warren-slams-big-agriculture-calls-to-reverse-mergers-to-aid-farmers.html [https://perma.cc/SRX2-3757]. ↑
- See supra Section I.A.2. ↑
- The USDA enforces pesticide residue limits in meat and poultry, while the FDA enforces these limits on all other food products. See Kate Z. Graham, Federal Regulation of Pesticide Residues: A Brief History and Analysis, 15 J. Food L. & Pol’y 98, 108, 118 (2019). ↑
- Rebecca Tushnet, Ellen Goodman & Samara Spence, SEC’s Climate Rule Is Compatible with the 1st Amendment, Law360 (July 28, 2022, 5:00 PM), https://www.law360.com/articles/1514053 [https://perma.cc/GN93-9JG7]. ↑
- Graham, supra note 303, at 108. ↑
- See generally id. (laying out the current federal regulatory scheme). ↑
- Sanne H. Knudsen, Regulating Cumulative Risk, 101 Minn. L. Rev. 2313, 2351 (2017). ↑
- Even outside of the science context, Americans rank “fake news” as a bigger issue than violent crime, climate change, terrorism, and more. Amy Mitchell et al., Many Americans Say Made-Up News Is a Critical Problem that Needs to be Fixed, Pew Rsch. Ctr. (June 5, 2019), https://www.pewresearch.org/journalism/2019/06/05/many-americans-say-made-up-news-is-a-critical-problem-that-needs-to-be-fixed [https://perma.cc/FDQ2-MCA8]. ↑
